Protective Effects of Acetylation on the Pathological Reactions of the Lens Crystallins with Homocysteine Thiolactone
- PMID: 27706231
- PMCID: PMC5051903
- DOI: 10.1371/journal.pone.0164139
Protective Effects of Acetylation on the Pathological Reactions of the Lens Crystallins with Homocysteine Thiolactone
Abstract
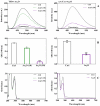
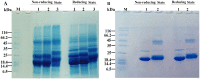
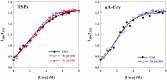
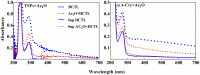
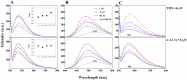
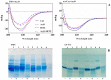
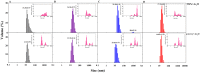
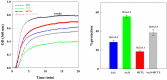
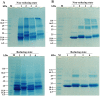
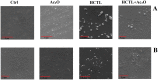
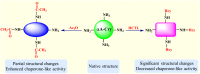
Various post-translational lens crystallins modifications result in structural and functional insults, contributing to the development of lens opacity and cataract disorders. Lens crystallins are potential targets of homocysteinylation, particularly under hyperhomocysteinemia which has been indicated in various eye diseases. Since both homocysteinylation and acetylation primarily occur on protein free amino groups, we applied different spectroscopic methods and gel mobility shift analysis to examine the possible preventive role of acetylation against homocysteinylation. Lens crystallins were extensively acetylated in the presence of acetic anhydride and then subjected to homocysteinylation in the presence of homocysteine thiolactone (HCTL). Extensive acetylation of the lens crystallins results in partial structural alteration and enhancement of their stability, as well as improvement of α-crystallin chaperone-like activity. In addition, acetylation partially prevents HCTL-induced structural alteration and aggregation of lens crystallins. Also, acetylation protects against HCTL-induced loss of α-crystallin chaperone activity. Additionally, subsequent acetylation and homocysteinylation cause significant proteolytic degradation of crystallins. Therefore, further experimentation is required in order to judge effectively the preventative role of acetylation on the structural and functional insults induced by homocysteinylation of lens crystallins.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Bhat SP, Nagineni CN. Alpha B subunit of lens-specific protein alpha crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989; 158: 319–325. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

