Novel Hydrophobin Fusion Tags for Plant-Produced Fusion Proteins
- PMID: 27706254
- PMCID: PMC5051927
- DOI: 10.1371/journal.pone.0164032
Novel Hydrophobin Fusion Tags for Plant-Produced Fusion Proteins
Abstract
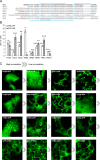
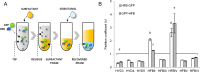
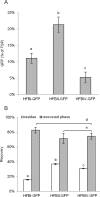
Hydrophobin fusion technology has been applied in the expression of several recombinant proteins in plants. Until now, the technology has relied exclusively on the Trichoderma reesei hydrophobin HFBI. We screened eight novel hydrophobin tags, T. reesei HFBII, HFBIII, HFBIV, HFBV, HFBVI and Fusarium verticillioides derived HYD3, HYD4 and HYD5, for production of fusion proteins in plants and purification by two-phase separation. To study the properties of the hydrophobins, we used N-terminal and C-terminal GFP as a fusion partner. Transient expression of the hydrophobin fusions in Nicotiana benthamiana revealed large variability in accumulation levels, which was also reflected in formation of protein bodies. In two-phase separations, only HFBII and HFBIV were able to concentrate GFP into the surfactant phase from a plant extract. The separation efficiency of both tags was comparable to HFBI. When the accumulation was tested side by side, HFBII-GFP gave a better yield than HFBI-GFP, while the yield of HFBIV-GFP remained lower. Thus we present here two alternatives for HFBI as functional fusion tags for plant-based protein production and first step purification.
Conflict of interest statement
All authors are, or have been, employed by VTT Technical research Centre of Finland Ltd. VTT owns a patent (WO 2000058342 A1, Process for partitioning of proteins) related to hydrophobin fusion technology. There are no further patents, products in development or marketed products to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Hydrophobin fusions for high-level transient protein expression and purification in Nicotiana benthamiana.Plant Physiol. 2010 Feb;152(2):622-33. doi: 10.1104/pp.109.149021. Epub 2009 Dec 11. Plant Physiol. 2010. PMID: 20018596 Free PMC article.
-
[Effect of the hydrophobin HFBI-fusion tag on exogenous protein accumulation in tobacco plant].Nan Fang Yi Ke Da Xue Xue Bao. 2015 Dec;35(12):1665-71. Nan Fang Yi Ke Da Xue Xue Bao. 2015. PMID: 26714894 Chinese.
-
Protein body formation in stable transgenic tobacco expressing elastin-like polypeptide and hydrophobin fusion proteins.BMC Biotechnol. 2013 May 10;13:40. doi: 10.1186/1472-6750-13-40. BMC Biotechnol. 2013. PMID: 23663656 Free PMC article.
-
Green biofactories: recombinant protein production in plants.Recent Pat Biotechnol. 2010 Nov;4(3):242-59. Recent Pat Biotechnol. 2010. PMID: 21171961 Review.
-
Protein body-inducing fusions for high-level production and purification of recombinant proteins in plants.Plant Biotechnol J. 2011 May;9(4):419-33. doi: 10.1111/j.1467-7652.2011.00596.x. Epub 2011 Feb 22. Plant Biotechnol J. 2011. PMID: 21338467 Review.
Cited by
-
Transient Expression of Zika NS2B Protein Antigen in Nicotiana benthamiana and Use for Arboviruses Diagnosis.ACS Omega. 2025 Jan 8;10(2):2184-2196. doi: 10.1021/acsomega.4c08998. eCollection 2025 Jan 21. ACS Omega. 2025. PMID: 39866622 Free PMC article.
-
Hydrophobin-Fused SARS-CoV-2 RBD production in Nicotiana benthamiana L. for COVID-19 serology.Future Sci OA. 2025 Dec;11(1):2527502. doi: 10.1080/20565623.2025.2527502. Epub 2025 Jul 4. Future Sci OA. 2025. PMID: 40613498 Free PMC article.
-
Cytosolic and Nuclear Co-localization of Betalain Biosynthetic Enzymes in Tobacco Suggests that Betalains Are Synthesized in the Cytoplasm and/or Nucleus of Betalainic Plant Cells.Front Plant Sci. 2017 May 18;8:831. doi: 10.3389/fpls.2017.00831. eCollection 2017. Front Plant Sci. 2017. PMID: 28572813 Free PMC article.
-
Subcellular Localization of a Plant Catalase-Phenol Oxidase, AcCATPO, from Amaranthus and Identification of a Non-canonical Peroxisome Targeting Signal.Front Plant Sci. 2017 Aug 2;8:1345. doi: 10.3389/fpls.2017.01345. eCollection 2017. Front Plant Sci. 2017. PMID: 28824680 Free PMC article.
-
Hydrophobins: multifunctional biosurfactants for interface engineering.J Biol Eng. 2019 Jan 23;13:10. doi: 10.1186/s13036-018-0136-1. eCollection 2019. J Biol Eng. 2019. PMID: 30679947 Free PMC article. Review.
References
-
- Varjonen S, Laaksonen P, Paananen A, Valo H, Hähl H, Laaksonen T, et al. Self-assembly of cellulose nanofibrils by genetically engineered fusion proteins. Soft Matter. Royal Society of Chemistry; 2011. March 7;7(6):2402 10.1039/c0sm01114b - DOI
-
- Cox AR, Aldred DL, Russell AB. Exceptional stability of food foams using class II hydrophobin HFBII. Food Hydrocoll. 2009. March;23(2):366–76. 10.1016/j.foodhyd.2008.03.001 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

