The Effect of Albumin on MRP2 and BCRP in the Vesicular Transport Assay
- PMID: 27706255
- PMCID: PMC5051865
- DOI: 10.1371/journal.pone.0163886
The Effect of Albumin on MRP2 and BCRP in the Vesicular Transport Assay
Abstract
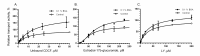
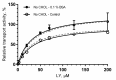
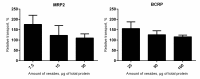
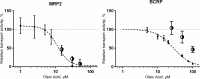
The ABC transporters multidrug resistance associated protein 2 (MRP2) and breast cancer resistance protein (BCRP) are of interest in drug development, since they affect the pharmacokinetics of several drugs. Membrane vesicle transport assays are widely used to study interactions with these proteins. Since albumin has been found to affect the kinetics of metabolic enzymes in similar membrane preparations, we investigated whether albumin affects the kinetic parameters of efflux transport. We found that albumin increased the Vmax of 5(6)-carboxy-2',7'-dichlorofluorescein (CDCF) and estradiol-17-β-D-glucuronide uptake into MRP2 vesicles in the presence of 0.1% bovine serum albumin (BSA) by 2 and 1.5-fold, respectively, while BSA increased Lucifer yellow uptake by 30% in BCRP vesicles. Km values increased slightly, but the change was not statistically significant. The effect of BSA on substrate uptake was dependent on the vesicle amount, while increasing BSA concentration did not significantly improve substrate uptake. These results indicate a minor effect of albumin on MRP2 and BCRP, but it should be considered if albumin is added to transporter assays for example as a solubilizer, since the effect may be substrate or transporter specific.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
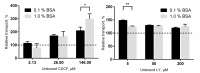
References
-
- Schaub TP, Kartenbeck J, Konig J, Spring H, Dorsam J, Staehler G, et al. Expression of the MRP2 gene-encoded conjugate export pump in human kidney proximal tubules and in renal cell carcinoma. J Am Soc Nephrol. 1999;10(6):1159–69. - PubMed
-
- Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61(8):3458–64. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

