Bisphosphonates for steroid-induced osteoporosis
- PMID: 27706804
- PMCID: PMC6461188
- DOI: 10.1002/14651858.CD001347.pub2
Bisphosphonates for steroid-induced osteoporosis
Abstract
Background: This is an update of a Cochrane Review first published in 1999. Corticosteroids are widely used in inflammatory conditions as an immunosuppressive agent. Bone loss is a serious side effect of this therapy. Several studies have examined the use of bisphosphonates in the prevention and treatment of glucocorticosteroid-induced osteoporosis (GIOP) and have reported varying magnitudes of effect.
Objectives: To assess the benefits and harms of bisphosphonates for the prevention and treatment of GIOP in adults.
Search methods: We searched CENTRAL, MEDLINE and Embase up to April 2016 and International Pharmaceutical Abstracts (IPA) via OVID up to January 2012 for relevant articles and conference proceedings with no language restrictions. We searched two clinical trial registries for ongoing and recently completed studies (ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal). We also reviewed reference lists of relevant review articles.
Selection criteria: We included randomised controlled trials (RCTs) satisfying the following criteria: 1) prevention or treatment of GIOP; 2) adults taking a mean steroid dose of 5.0 mg/day or more; 3) active treatment including bisphosphonates of any type alone or in combination with calcium or vitamin D; 4) comparator treatment including a control of calcium or vitamin D, or both, alone or with placebo; and 4) reporting relevant outcomes. We excluded trials that included people with transplant-associated steroid use.
Data collection and analysis: At least two review authors independently selected trials for inclusion, extracted data, performed 'risk of bias' assessment and evaluated the certainty of evidence using the GRADE approach. Major outcomes of interest were the incidence of vertebral and nonvertebral fractures after 12 to 24 months; the change in bone mineral density (BMD) at the lumbar spine and femoral neck after 12 months; serious adverse events; withdrawals due to adverse events; and quality of life. We used standard Cochrane methodological procedures.
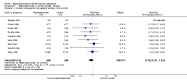
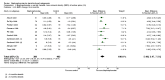
Main results: We included a total of 27 RCTs with 3075 participants in the review. Pooled analysis for incident vertebral fractures included 12 trials (1343 participants) with high-certainty evidence and low risk of bias. In this analysis 46/597 (or 77 per 1000) people experienced new vertebral fractures in the control group compared with 31/746 (or 44 per 1000; range 27 to 70) in the bisphosphonate group; relative improvement of 43% (9% to 65% better) with bisphosphonates; absolute increased benefit of 2% fewer people sustaining fractures with bisphosphonates (5% fewer to 1% more); number needed to treat for an additional beneficial outcome (NNTB) was 31 (20 to 145) meaning that approximately 31 people would need to be treated with bisphosphonates to prevent new vertebral fractures in one person.Pooled analysis for incident nonvertebral fractures included nine trials with 1245 participants with low-certainty evidence (downgraded for imprecision and serious risk of bias as a patient-reported outcome). In this analysis 30/546 (or 55 per 1000) people experienced new nonvertebral fracture in the control group compared with 29/699 (or 42 per 1000; range 25 to 69) in the bisphosphonate group; relative improvement of 21% with bisphosphonates (33% worse to 53% better); absolute increased benefit of 1% fewer people with fractures with bisphosphonates (4% fewer to 1% more).Pooled analysis on BMD change at the lumbar spine after 12 months included 23 trials with 2042 patients. Eighteen trials with 1665 participants were included in the pooled analysis on BMD at the femoral neck after 12 months. Evidence for both outcomes was moderate-certainty (downgraded for indirectness as a surrogate marker for osteoporosis) with low risk of bias. Overall, the bisphosphonate groups reported stabilisation or increase in BMD, while the control groups showed decreased BMD over the study period. At the lumbar spine, there was an absolute increase in BMD of 3.5% with bisphosphonates (2.90% to 4.10% higher) with a relative improvement of 1.10% with bisphosphonates (0.91% to 1.29%); NNTB 3 (2 to 3). At the femoral neck, the absolute difference in BMD was 2.06% higher in the bisphosphonate group compared to the control group (1.45% to 2.68% higher) with a relative improvement of 1.29% (0.91% to 1.69%); NNTB 5 (4 to 7).Pooled analysis on serious adverse events included 15 trials (1703 participants) with low-certainty evidence (downgraded for imprecision and risk of bias). In this analysis 131/811 (or 162 per 1000) people experienced serious adverse events in the control group compared to 136/892 (or 147 per 1000; range 120 to 181) in the bisphosphonate group; absolute increased harm of 0% more serious adverse events (2% fewer to 2% more); a relative per cent change with 9% improvement (12% worse to 26% better).Pooled analysis for withdrawals due to adverse events included 15 trials (1790 patients) with low-certainty evidence (downgraded for imprecision and risk of bias). In this analysis 63/866 (or 73 per 1000) people withdrew in the control group compared to 76/924 (or 77 per 1000; range 56 to 107) in the bisphosphonate group; an absolute increased harm of 1% more withdrawals with bisphosphonates (95% CI 1% fewer to 3% more); a relative per cent change 6% worse (95% CI 47% worse to 23% better).Quality of life was not assessed in any of the trials.
Authors' conclusions: There was high-certainty evidence that bisphosphonates are beneficial in reducing the risk of vertebral fractures with data extending to 24 months of use. There was low-certainty evidence that bisphosphonates may make little or no difference in preventing nonvertebral fractures. There was moderate-certainty evidence that bisphosphonates are beneficial in preventing and treating corticosteroid-induced bone loss at both the lumbar spine and femoral neck. Regarding harm, there was low-certainty evidence that bisphosphonates may make little or no difference in the occurrence of serious adverse events or withdrawals due to adverse events. We are cautious in interpreting these data as markers for harm and tolerability due to the potential for bias.Overall, our review supports the use of bisphosphonates to reduce the risk of vertebral fractures and the prevention and treatment of steroid-induced bone loss.
Conflict of interest statement
Claire S Allen: none known James HS Yeung: none known Ben Vandermeer: none known Joanne Homik: none known
Figures

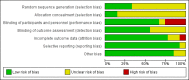
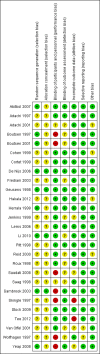











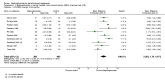


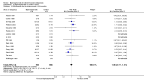
Update of
-
Bisphosphonates for steroid induced osteoporosis.Cochrane Database Syst Rev. 2000;(2):CD001347. doi: 10.1002/14651858.CD001347. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2016 Oct 05;10:CD001347. doi: 10.1002/14651858.CD001347.pub2. PMID: 10796432 Updated.
References
References to studies included in this review
Abitbol 2007 {published data only}
-
- Abitbol V, Briot K, Roux C, Roy C, Seksik P, Charachon A, et al. A double‐blind placebo‐controlled study of intravenous clodronate for prevention of steroid‐induced bone loss in inflammatory bowel disease. Clinical Gastroenterology & Hepatology 2007;5:1184‐9. - PubMed
Adachi 1997 {published data only}
-
- Adachi JD, Bensen WG, Brown J, Hanley D, Hodsman A, Josse R, et al. Intermittent etidronate therapy to prevent corticosteroid‐induced osteoporosis. New England Journal of Medicine 1997;337(6):382‐7. - PubMed
Adachi 2001 {published data only}
-
- Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, et al. Two‐year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double‐blind, placebo‐controlled extension trial. Arthritis & Rheumatism 2001;44:202‐11. - PubMed
Boutsen 1997 {published data only}
-
- Boutsen Y, Jamart J, Esselinckx W, Stoffel M, Devogelaer JP. Primary prevention of glucocorticoid‐induced osteoporosis with intermittent intravenous pamidronate: a randomized trial. Calcified Tissue International 1997;61:266‐71. - PubMed
Boutsen 2001 {published data only}
-
- Boutsen Y, Jamart J, Esselinckx W, Devogelaer JP. Primary prevention of glucocorticoid‐induced osteoporosis with intravenous pamidronate and calcium: a prospective controlled 1‐year study comparing a single infusion, an infusion given once every 3 months, and calcium alone. Journal of Bone & Mineral Research 2001;16:104‐12. - PubMed
Cohen 1999 {published data only}
-
- Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, et al. Risedronate therapy prevents corticosteroid‐induced bone loss: a twelve‐month, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Arthritis & Rheumatism 1999;42:2309‐18. - PubMed
Cortet 1999 {published data only}
-
- Cortet B, Hachulla E, Barton I, Bonvoisin B, Roux C. Evaluation of the efficacy of etidronate therapy in preventing glucocorticoid‐induced bone loss in patients with inflammatory rheumatic diseases. A randomized study. Revue du Rhumatisme (English Edition) 1999;66:214‐9. - PubMed
De Nijs 2006 {published data only}
-
- Nijs RN, Jacobs JW, Lems WF, Laan RF, Algra A, Huisman AM, et al. Alendronate or alfacalcidol in glucocorticoid‐induced osteoporosis.[Reprint in Ned Tijdschr Geneeskd. 2007 May 26;151(21):1178‐85; PMID: 17557758]. New England Journal of Medicine 2006;355:675‐84.
Frediani 2003 {published data only}
-
- Frediani B, Falsetti P, Baldi F, Acciai C, Filippou G, Marcolongo R. Effects of 4‐year treatment with once‐weekly clodronate on prevention of corticosteroid‐induced bone loss and fractures in patients with arthritis: evaluation with dual‐energy X‐ray absorptiometry and quantitative ultrasound. Bone 2003;33:575‐81. - PubMed
Geusens 1998 {published data only}
-
- Geusens P, Dequeker J, Vanhoof J, Stalmans R, Boonen S, Joly J, et al. Cyclical etidronate increases bone density in the spine and hip of postmenopausal women receiving long term corticosteroid treatment. A double blind, randomised placebo controlled study. Annals of the Rheumatic Diseases 1998;57:724‐7. - PMC - PubMed
Hakala 2012 {published data only}
-
- Hakala M, Kröger H, Valleala H, Hienonen‐Kempas T, Lehtonen‐Veromaa M, Heikkinen J, et al. Once‐monthly oral ibandronate provides significant improvement in bone mineral density in postmenopausal women treated with glucocorticoids for inflammatory rheumatic diseases: a 12‐month, randomized, double‐blind, placebo‐controlled trial. Scandinavian Journal of Rheumatology 2012;41(4):260‐6. - PubMed
Herrala 1998 {published data only}
-
- Herrala J, Puolijoki H, Liippo K, Raitio M, Impivaara O, Tala E, et al. Clodronate is effective in preventing corticosteroid‐induced bone loss among asthmatic patients. Bone 1998;22:577‐82. - PubMed
Jenkins 1999 {published data only}
-
- Jenkins EA, Walker‐Bone KE, Wood A, McCrae FC, Cooper C, Cawley MI. The prevention of corticosteroid‐induced bone loss with intermittent cyclical etidronate. Scandinavian Journal of Rheumatology 1999;28:152‐6. - PubMed
Lems 2006 {published data only}
-
- Lems WF, Lodder MC, Lips P, Bijlsma JW, Geusens P, Schrameijer N, et al. Positive effect of alendronate on bone mineral density and markers of bone turnover in patients with rheumatoid arthritis on chronic treatment with low‐dose prednisone: a randomized, double‐blind, placebo‐controlled trial. Osteoporosis International 2006;17:716‐23. - PubMed
Li 2010 {published data only}
Pitt 1998 {published data only}
Reid 2000 {published data only}
-
- Reid DM, Hughes RA, Laan RF, Sacco‐Gibson NA, Wenderoth DH, Adami S, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid‐induced osteoporosis in men and women: a randomized trial. European Corticosteroid‐Induced Osteoporosis Treatment Study. Journal of Bone & Mineral Research 2000;15:1006‐13. - PubMed
Roux 1998 {published data only}
-
- Roux C, Oriente P, Laan R, Hughes RA, Ittner J, Goemaere S, et al. Randomized trial of effect of cyclical etidronate in the prevention of corticosteroid‐induced bone loss. Ciblos Study Group. Journal of Clinical Endocrinology & Metabolism 1998;83:1128‐33. - PubMed
Saadati 2008 {published data only}
-
- Saadati N, Rajabian R. The effect of bisphosphonate on prevention of glucocorticoid‐induced osteoporosis. Iranian Red Crescent Medical Journal 2008;10(1):8‐11.
Saag 1998 {published data only}
-
- Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, et al. Alendronate for the prevention and treatment of glucocorticoid‐induced osteoporosis. New England Journal of Medicine 1998;339:292‐9. - PubMed
Sambrook 2003 {published data only}
-
- Sambrook PN, Kotowicz M, Nash P, Styles CB, Naganathan V, Henderson‐Briffa KN, et al. Prevention and treatment of glucocorticoid‐induced osteoporosis: a comparison of calcitriol, vitamin D plus calcium, and alendronate plus calcium. Journal of Bone & Mineral Research 2003;18:919‐24. - PubMed
Skingle 1997 {published data only}
-
- Skingle SJ, Moore DJ, Crisp AJ. Cyclical etidronate increases lumbar spine bone density in patients on long‐term glucocorticosteroid therapy. International journal of clinical practice 1997;51:364‐7. - PubMed
Stoch 2009 {published data only}
-
- Stoch SA, Saag KG, Greenwald M, Sebba AI, Cohen S, Verbruggen N, et al. Once‐weekly oral alendronate 70 mg in patients with glucocorticoid‐induced bone loss: a 12‐month randomized, placebo‐controlled clinical trial. Journal of Rheumatology 2009;36:1705‐14. - PubMed
Tee 2012 {published data only}
-
- Tee SI, Yosipovitch G, Chan YC, Chua SH, Koh ET, Chan YH, et al. Prevention of glucocorticoid‐induced osteoporosis in immunobullous diseases with alendronate: a randomized, double‐blind, placebo‐controlled study. Archives of Dermatology 2012;148(3):307‐14. - PubMed
Van Offel 2001 {published data only}
-
- Offel JF, Schuerwegh AJ, Bridts CH, Bracke PG, Stevens WJ, Clerck LS. Influence of cyclic intravenous pamidronate on proinflammatory monocytic cytokine profiles and bone density in rheumatoid arthritis treated with low dose prednisolone and methotrexate. Clinical & Experimental Rheumatology 2001;19:13‐20. - PubMed
Wolfhagen 1997 {published data only}
-
- Wolfhagen FH, Buuren HR, Ouden JW, Hop WC, Leeuwen JP, Schalm SW, et al. Cyclical etidronate in the prevention of bone loss in corticosteroid‐treated primary biliary cirrhosis. A prospective, controlled pilot study. Journal of Hepatology 1991;26:325‐30. - PubMed
Yeap 2008 {published data only}
-
- Yeap SS, Fauzi AR, Kong NC, Halim AG, Soehardy Z, Rahimah I, et al. A comparison of calcium, calcitriol, and alendronate in corticosteroid‐treated premenopausal patients with systemic lupus erythematosus. Journal of Rheumatology 2008;35:2344‐7. - PubMed
References to studies excluded from this review
Benucci 2009 {published data only}
-
- Benucci M, Saviola G, Baiardi P, Abdi‐Ali L, Povino MR, Dolenti S, et al. Effects of monthly intramuscular neridronate in rheumatic patients in chronic treatment with low‐dose glucocorticoids. Clinical and Experimental Rheumatology 2009;27(4):567‐73. - PubMed
Fujii 2006 {published data only}
-
- Fujii N, Hamano T, Mikami S, Nagasawa Y, Isaka Y, Moriyama T, et al. Risedronate, an effective treatment for glucocorticoid‐induced bone loss in CKD patients with or without concomitant active vitamin D (PRIUS‐CKD). Nephrology Dialysis Transplantation 2007;22:1601‐7. - PubMed
Jinnouchi 2000 {published data only}
-
- Jinnouchi Y. Efficacy of intermittent etidronate therapy for corticosteroid‐induced osteoporosis in patients with diffuse connective tissue disease. Kurume Medical Journal 2000;47:219‐24. - PubMed
Kikuchi 2006 {published data only}
-
- Kikuchi Y, Imakiire T, Yamada M, Saigusa T, Hyodo T, Kushiyama T, et al. Effect of risedronate on high‐dose corticosteroid‐induced bone loss in patients with glomerular disease. Nephrology Dialysis Transplantation 2007;22:1593‐600. - PubMed
Kitazaki 2008 {published data only}
-
- Kitazaki S, Mitsuyama K, Masuda J, Harada K, Yamasaki H, Kuwaki K, et al. Clinical trial: comparison of alendronate and alfacalcidol in glucocorticoid‐associated osteoporosis in patients with ulcerative colitis. Alimentary Pharmacology & Therapeutics 2009;29(4):424‐30. - PubMed
Nakayamada 2004 {published data only}
-
- Nakayamada S, Okada Y, Saito K, Tanaka Y. Etidronate prevents high dose glucocorticoid induced bone loss in premenopausal individuals with systemic autoimmune diseases. Journal of Rheumatology 2004;31:163‐6. - PubMed
Okada 2008 {published data only}
-
- Okada Y, Nawata M, Nakayamada S, Saito K, Tanaka Y. Alendronate protects premenopausal women from bone loss and fracture associated with high‐dose glucocorticoid therapy. Journal of Rheumatology 2008;35:2249‐54. - PubMed
Sato 2003 {published data only}
-
- Sato S, Ohosone Y, Suwa A, Yasuoka H, Nojima T, Fujii T, et al. Effect of intermittent cyclical etidronate therapy on corticosteroid induced osteoporosis in Japanese patients with connective tissue disease: 3 year follow up. Journal of Rheumatology 2003;30:2673‐9. - PubMed
Takeda 2008 {published data only}
-
- Takeda S, Kaneoka H, Saito T. Effect of alendronate on glucocorticoid‐induced osteoporosis in Japanese women with systemic autoimmune diseases: versus alfacalcidol. Modern Rheumatology 2008;18(3):271‐6. - PubMed
Takei 2010 {published data only}
-
- Takei T, Itabashi M, Tsukada M, Sugiura H, Moriyama T, Kojima C, et al. Risedronate therapy for the prevention of steroid‐induced osteoporosis in patients with minimal‐change nephrotic syndrome. Internal Medicine 2010;49(19):2065‐70. - PubMed
Toukap 2005 {published data only}
-
- Toukap AN, Depresseux G, Devogelaer JP, Houssiau FA. Oral pamidronate prevents high‐dose glucocorticoid‐induced lumbar spine bone loss in premenopausal connective tissue disease (mainly lupus) patients. Lupus 2005;14:517‐20. - PubMed
References to studies awaiting assessment
Imanishi 2006 {published data only}
-
- Imanishi Y, Nishizawa Y. Activate form vitamin D3 or bisphosphonate in glucocorticoid‐induced osteoporosis. Clinical Calcium 2006;16(11):1844‐50. - PubMed
Nakamura 2002 {published data only}
-
- Nakamura T, Maekawa S, Morinobu S, Morinobu A, Koshiba M, Yamauchi M, et al. The clinical benefits to bone mineral density were shown by cyclical oral etidronate administration in steroid induced osteoporosis. Ryumachi 2002;42(4):666‐75. - PubMed
Okazaki 2015 {published data only}
-
- Okazaki, R. Pharmacological treatment of other types of secondary osteoporosis. Nippon Rinsho ‐ Japanese Journal of Clinical Medicine 2015;73(10):1740‐5. - PubMed
Ozoran 2007 {published data only}
-
- Ozoran K, Yildirim M, Önder M, Sivas F, Inanir A. The bone mineral density effects of calcitonin and alendronate combined therapy in patients with rheumatoid arthritis. Asia Pacific League of Associations for Rheumatology Journal of Rheumatology 2007;10:17‐22.
Suzuki 2015 {published data only}
-
- Suzuki Y. Glucocorticoid‐induced osteoporosis. Nippon Rinsho ‐ Japanese Journal of Clinical Medicine 2015;73(10):1733‐39. - PubMed
References to ongoing studies
NCT00058188 {unpublished data only}
-
- A phase III randomized study of zoledronate bisphosphonate therapy for the prevention of bone loss in men with prostate cancer receiving long‐term androgen deprivation. Ongoing study March 2003.
NCT02589600 {unpublished data only}
-
- ZEST II for osteoporotic fracture prevention. Ongoing study January 2016.
UMIN000009222 {unpublished data only}
-
- Drug therapy for the prevention of glucocorticoid induced osteoporosis in elderly patients: teriparatide or bisphosphonates?. Ongoing study 2012/12/01.
UMIN000013305 {unpublished data only}
-
- Efficacy of once every four week oral minodronate in patients with glucocorticoid‐induced osteoporosis after switching from weekly oral bisphosphonate. Ongoing study 2013/10/25.
UMIN000013659 {unpublished data only}
-
- Efficacy of a human anti‐RANKL antibody (Denosumab) on prevention of steroid‐induced osteoporosis in patients with autoimmune hepatitis (AIH). Ongoing study 2014/04/08.
UMIN000014341 {unpublished data only}
-
- Glucocorticoid‐induced osteoporosis treated with bisphosphonate and denosumab. Ongoing study 2014/06/24.
Additional references
Abrahamsen 2010
Black 1996
-
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt M C, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996;348:1535‐41. - PubMed
Brant 2014 [Computer program]
-
- Brant, R. Inference for Proportions: Comparing Two Independent Samples. Accessed June 12 2015: stat.ubc.ca/˜rollin/stats/ssize/b2.html, 2014.
Canalis 2007
-
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid‐induced osteoporosis: pathophysiology and therapy. Osteoporosis International 2007;18:1319‐28. - PubMed
Cates 2015 [Computer program]
-
- Cates C. Visual Rx Software. 2008. Version Version 1.0 ‐ 3.0. Available from nntonline.net/visualrx/, accessed June 12, 2015.
Cooper 1992
-
- Cooper C, Atkinson EJ, O'Fallon WM, Melton JK 3rd. Incidence of clinically diagnosed vertebral fractures: a population‐based study in Rochester, Minnesota, 1985‐1989. Journal of Bone and Mineral Research 1992;7:221‐7. - PubMed
Cummings 2002
-
- Cummings, S. R, Karpf, D. B, Harris, F, Genant, H. K, Ensrud, K, LaCroix, A. Z, et al. Improvement in Spine Bone Density and Reduction in Risk of Vertebral Fractures during Treatment with Antiresorptive Drugs. American Journal of Medicine 2002;112:281‐9. - PubMed
Curtis 2005
-
- Curtis JR, Westfall AO, Allison JJ, Becker A, Casebeer L, Freeman A, et al. Longitudinal patterns in the prevention of osteoporosis in glucocorticoid‐treated patients. Arthritis & Rheumatism 2005;52(8):2485‐94. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dell 2012
-
- Dell R, Greene D, Ott SM, Silverman S, Eisemon E, Funahashi T, et al. A retrospective analysis of all atypical femur fractures seen in a large California HMO from the years 2007 to 2009. Journal of Bone and Mineral Research 2012;27(12):2544–50. - PubMed
Djokanovic 2008
-
- Djokanovic N, Klieger‐Grossmann C, Koren G. Does treatment with bisphosphonates endanger the human pregnancy?. Journal of Obstetrics and Gynaecology Canada 2008;30(12):1146‐8. - PubMed
Feldstein 2005
-
- Feldstein AC, Elmer PJ, Nichols GA, Herson M. Practice patterns in patients at risk for glucocorticoid‐induced osteoporosis. Osteoporosis International 2005;16(12):2168‐74. - PubMed
Genant 1993
-
- Genant HK, Wu CY, Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. Journal of Bone and Mineral Research 1993;8(9):1137‐48. - PubMed
Genant 1996
-
- Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis The Study of Osteoporotic Fractures Research Group. Journal of Bone and Mineral Research 1996;11(7):984‐96. - PubMed
Gertz 1995
-
- Gertz BJ, Holland SD, Kline WF, Matuszewski BK, Freeman A, Quan H, et al. Studies on the oral availablility of alendronate. Clinical Pharmacology & Therapeutics 1995;58(3):288‐98. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 12 June 2015. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Grossman 2010
-
- Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid‐induced osteoporosis. Arthritis Care and Research 2010;62(11):1515‐26. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kanis 2007
-
- Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd‐Jones M. Glucocorticoid‐induced osteoporosis: a systematic review and cost–utility analysis. Health Technology Assessment 2007;11:7. - PubMed
Kiel 1995
-
- Kiel, D. Assessing vertebral fractures: National Osteoporosis Working Group on Vertebral Fractures. Journal of Bone and Mineral Research 1995;10:518–23. - PubMed
Kleerekoper 1984
-
- Kleerekoper M, Parfitt AM, Ellis BI. Measurements of vertebral fracture rates in osteoporosis. Proceedings of the Copenhagen International Symposium on Osteoporosis. Copenhagen: AalbergStiftsbogtrykkeri, June 3‐8, 1984:103‐109.
Lee 2015
-
- Lee S, Yin RV, Hirpara H, Lee NC, Lee A, Llanos S, et al. Increased risk for atypical fractures associated with bisphosphonate use. Family Practice 2015;32(3):276–81. - PubMed
Lekamwasam 2012
-
- Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgström F, et al. A framework for the development of guidelines for the management of glucocorticoid‐induced osteoporosis. Osteoporosis International 2012;23(9):2257‐76. - PubMed
Losada 2010
-
- Losada I, Sartori L, Gianantonio E, Zen M, Clementi M, Doria A. Bisphosphonates in patients with autoimmune rheumatic diseases: can they be used in women of childbearing age?. Autoimmunity Reviews 2010;9(8):547‐52. - PubMed
Mckeown 2012
-
- McKeown E, Vivian P, Bykerk VP, Leon F, Bonner A, Thorne C, et al. Quality assurance study of the use of preventative therapies in glucocorticoid‐induced osteoporosis in early inflammatory arthritis: results from the CATCH cohort. Rheumatology 2012;51:1662‐9. - PubMed
Melton 1993
-
- Melton LJ 3rd, Lane AW, Eastell R, O’Fallon WM, Riggs BL. Prevalence and incidence of vertebral deformities. Osteoporosis International 1993;3:113‐9. - PubMed
Minne 1988
-
- Minne HW, Leidig G, Wuster C, Siromachkostov L, Baldauf G, Bickel R, et al. A newly developed spine deformity index (SDI) to quantitate vertebral crush fractures in patients with osteoporosis. Bone Mineral 1988;3:335–49. - PubMed
National Osteoporosis Foundation 2014
Pazianas 2011
-
- Pazianas M, Abrahamsen B. Safety of Bisphosphonates. Bone 2011;49:103–10. - PubMed
Reid 2009
-
- Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid‐induced osteoporosis (HORIZON): a multicentre, double‐blind, double‐dummy, randomised controlled trial. Lancet 2009;373:1253–63. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rickers 1984
-
- Rickers H, Deding A, Christiansen C, Rodbro P. Mineral loss in cortical and trabecular bone during high‐dose prednisone treatment. Calcified Tissue International 1984;36(3):269–73. - PubMed
Russell 2007
-
- Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Annals of the New York Academy of Sciences 2007;1117:209‐57. - PubMed
Saag 2003
-
- Saag KG. Glucocorticoid‐induced osteoporosis. Endocrinology and Metabolism Clinics of North America 2003;32:135‐57. - PubMed
Sambrook 2012
-
- Sambrook PN, Roux C, Devogelaer JP, Saag K, Lau CS, Reginster JY, et al. Bisphosphonates and glucocorticoid osteoporosis in men: results of a randomized controlled trial comparing zoledronic acid with risedronate. Bone 2012;50:289–95. - PubMed
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.handbook.cochrane.org.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, (editors). GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook.
Steinbuch 2004
-
- Steinbuch M, Thomas E, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporosis International 2004;15:323‐8. - PubMed
Van Kujik 1995
-
- Kuijk C, Genant HK. Radiological aspects. In: Riggs BL, Melton LJ III editor(s). Osteoporosis: etiology, diagnosis, and management. 2nd Edition. Philadelphia: Lippincott‐Raven, 1995:249‐73.
Van Staa 2002
-
- Staa TP, Leufkens HGM, Cooper C. The Epidemiology of Corticosteroid‐Induced Osteoporosis: a Meta‐analysis. Osteoporosis International 2002;13:777‐87. - PubMed
Wiebe 2006
-
- Wiebe N, Vandermeer B, Platt RW, Klassen TP, Moher D, Barrowman NJ. A systematic review identifies a lack of standardization in methods for handling missing variance data. Journal of Clinical Epidemiology 2006;59:342‐53. - PubMed
References to other published versions of this review
Homik 1999
Homik 1999a
-
- Homik JE, Cranney A, Shea B, Tugwell P, Wells G, Adachi JD, et al. A meta‐analysis on the use of bisphosphonates in corticosteroid induced osteoporosis. Journal of Rheumatology 1999;26(5):1148‐57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

