Sperm-Coating Beta-Defensin 126 Is a Dissociation-Resistant Dimer Produced by Epididymal Epithelium in the Bovine Reproductive Tract
- PMID: 27707712
- PMCID: PMC5333941
- DOI: 10.1095/biolreprod.116.138719
Sperm-Coating Beta-Defensin 126 Is a Dissociation-Resistant Dimer Produced by Epididymal Epithelium in the Bovine Reproductive Tract
Abstract
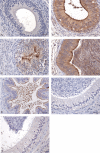
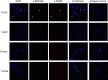
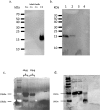
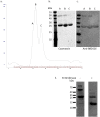
Beta-defensins are innate immune molecules, often described as antimicrobial peptides because of their bactericidal activity and are now known to have diverse additional functions, including cell signaling, chemoattraction, immunoregulation, and reproduction. In humans and primates, beta-defensin 126 has been shown to regulate the ability of sperm to swim through cervical mucus and to protect sperm from attack by the female immune system during transit toward the oviduct. Bovine beta-defensin 126 (BBD126) is the ortholog of human defensin 126, and computational analysis here revealed significant conservation between BBD126 and other mammalian orthologs at the N-terminus, although extensive sequence differences were detected at the C-terminus, implying possible species-specific roles for this beta-defensin in reproduction. We had previously demonstrated preferential expression of this and related beta-defensin genes in the bovine male reproductive tract, but no studies of bovine beta-defensin proteins have been performed to date. Here, we analyzed BBD126 protein using a monoclonal antibody (a-BBD126) generated against a 14 amino acid peptide sequence from the secreted fragment of BBD126. The specificity of a-BBD126 was validated by testing against the native form of the peptide recovered from bovine caudal epididymal fluid and recombinant BBD126 generated using a prokaryotic expression system. Western blot analysis of the native and recombinant forms showed that BBD126 exists as a dimer that was highly resistant to standard methods of dissociation. Immunohistochemical staining using a-BBD126 demonstrated BBD126 protein expression by epithelial cells of the caudal epididymis and vas deferens from both mature and immature bulls. BBD126 could also be seen (by confocal microscopy) to coat caudal sperm, with staining concentrated on the tail of the sperm cells. This study is the first to demonstrate beta-defensin 126 protein expression in the bovine reproductive tract and on bull sperm. Its dissociation-resistant dimeric structure is likely to have important functional implications for the role of BBD126 in bovine reproduction.
Keywords: bovine; epididymis; β-defensin 126.
© 2016 by the Society for the Study of Reproduction, Inc.
Figures

Similar articles
-
Cauda Epididymis-Specific Beta-Defensin 126 Promotes Sperm Motility but Not Fertilizing Ability in Cattle.Biol Reprod. 2016 Dec;95(6):122. doi: 10.1095/biolreprod.116.138792. Epub 2016 Oct 5. Biol Reprod. 2016. PMID: 27707713 Free PMC article.
-
Recombinant β-defensin 126 promotes bull sperm binding to bovine oviductal epithelia.Reprod Fertil Dev. 2018 Oct;30(11):1472-1481. doi: 10.1071/RD17415. Reprod Fertil Dev. 2018. PMID: 29773109
-
Beta-defensins as marker for male fertility: a comprehensive review†.Biol Reprod. 2023 Jan 14;108(1):52-71. doi: 10.1093/biolre/ioac197. Biol Reprod. 2023. PMID: 36322147 Review.
-
An epididymis-specific beta-defensin is important for the initiation of sperm maturation.Nat Cell Biol. 2004 May;6(5):458-64. doi: 10.1038/ncb1127. Nat Cell Biol. 2004. PMID: 15122269
-
A novel subclass of bovine β-defensins links reproduction and immunology.Reprod Fertil Dev. 2014;26(6):769-77. doi: 10.1071/RD13153. Reprod Fertil Dev. 2014. PMID: 23870162 Review.
Cited by
-
Cauda Epididymis-Specific Beta-Defensin 126 Promotes Sperm Motility but Not Fertilizing Ability in Cattle.Biol Reprod. 2016 Dec;95(6):122. doi: 10.1095/biolreprod.116.138792. Epub 2016 Oct 5. Biol Reprod. 2016. PMID: 27707713 Free PMC article.
-
β-Defensin gene copy number variation in cattle.R Soc Open Sci. 2024 Oct 30;11(10):241154. doi: 10.1098/rsos.241154. eCollection 2024 Oct. R Soc Open Sci. 2024. PMID: 39479249 Free PMC article.
-
Unusual interplay of contrasting selective pressures on β-defensin genes implicated in male fertility of the Buffalo (Bubalus bubalis).BMC Evol Biol. 2019 Nov 26;19(1):214. doi: 10.1186/s12862-019-1535-8. BMC Evol Biol. 2019. PMID: 31771505 Free PMC article.
-
Arl13b controls basal cell stemness properties and Hedgehog signaling in the mouse epididymis.Cell Mol Life Sci. 2022 Oct 19;79(11):556. doi: 10.1007/s00018-022-04570-1. Cell Mol Life Sci. 2022. PMID: 36261680 Free PMC article.
-
Buffalo sperm surface proteome profiling reveals an intricate relationship between innate immunity and reproduction.BMC Genomics. 2021 Jun 26;22(1):480. doi: 10.1186/s12864-021-07640-z. BMC Genomics. 2021. PMID: 34174811 Free PMC article.
References
-
- Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. - PubMed
-
- Tollner TL, Bevins CL, Cherr GN. Multifunctional glycoprotein DEFB126–a curious story of defensin-clad spermatozoa. Nat Rev Urol. 2012;9:365–375. - PubMed
-
- Tollner TL, Yudin AI, Treece CA, Overstreet JW, Cherr GN. Macaque sperm coating protein DEFB126 facilitates sperm penetration of cervical mucus. Hum Reprod. 2008;23:2523–2534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

