Typical gray matter axons in mammalian brain fail to conduct action potentials faithfully at fever-like temperatures
- PMID: 27707780
- PMCID: PMC5064137
- DOI: 10.14814/phy2.12981
Typical gray matter axons in mammalian brain fail to conduct action potentials faithfully at fever-like temperatures
Abstract
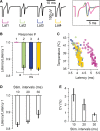
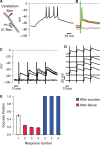
We studied the ability of typical unmyelinated cortical axons to conduct action potentials at fever-like temperatures because fever often gives CNS symptoms. We investigated such axons in cerebellar and hippocampal slices from 10 to 25 days old rats at temperatures between 30 and 43°C. By recording with two electrodes along axonal pathways, we confirmed that the axons were able to initiate action potentials, but at temperatures >39°C, the propagation of the action potentials to a more distal recording site was reduced. This temperature-sensitive conduction may be specific for the very thin unmyelinated axons because similar recordings from myelinated CNS axons did not show conduction failures. We found that the conduction fidelity improved with 1 mmol/L TEA in the bath, probably due to block of voltage-sensitive potassium channels responsible for the fast repolarization of action potentials. Furthermore, by recording electrically activated antidromic action potentials from the soma of cerebellar granule cells, we showed that the axons failed less if they were triggered 10-30 msec after another action potential. This was because individual action potentials were followed by a depolarizing after-potential, of constant amplitude and shape, which facilitated conduction of the following action potentials. The temperature-sensitive conduction failures above, but not below, normal body temperature, and the failure-reducing effect of the spike's depolarizing after-potential, are two intrinsic mechanisms in normal gray matter axons that may help us understand how the hyperthermic brain functions.
Keywords: Conduction failures; excitability; gray matter axons; presynaptic mechanisms.
© 2016 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures






References
-
- Adachi, R. , Yamada R., and Kuba H.. 2015. Plasticity of the axonal trigger zone. Neuroscientist 21:255–265. - PubMed
-
- Baginskas, A. , Palani D., Chiu K., and Raastad M.. 2009. The H‐current secures action potential transmission at high frequencies in rat cerebellar parallel fibers. Eur. J. Neurosci. 29:87–96. - PubMed
-
- Bernstein, J. 1902. Untersuchungen zur Thermodynamik der bioelektrischen Ströme. Pflügers Arch. 92:41.
-
- Blanton, M. G. , Lo Turco J. J., and Kriegstein A. R.. 1989. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J. Neurosci. Methods 30:203–210. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

