Arsenate reductase from Thermus thermophilus conjugated to polyethylene glycol-stabilized gold nanospheres allow trace sensing and speciation of arsenic ions
- PMID: 27707908
- PMCID: PMC5095221
- DOI: 10.1098/rsif.2016.0629
Arsenate reductase from Thermus thermophilus conjugated to polyethylene glycol-stabilized gold nanospheres allow trace sensing and speciation of arsenic ions
Abstract
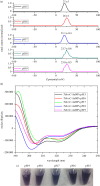
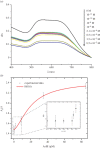
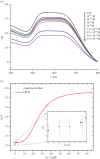
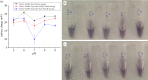
Water sources pollution by arsenic ions is a serious environmental problem all around the world. Arsenate reductase enzyme (TtArsC) from Thermus thermophilus extremophile bacterium, naturally binds arsenic ions, As(V) and As (III), in aqueous solutions. In this research, TtArsC enzyme adsorption onto hybrid polyethylene glycol-stabilized gold nanoparticles (AuNPs) was studied at different pH values as an innovative nanobiosystem for metal concentration monitoring. Characterizations were performed by UV/Vis and circular dichroism spectroscopies, TEM images and in terms of surface charge changes. The molecular interaction between arsenic ions and the TtArsC-AuNPs nanobiosystem was also monitored at all pH values considered by UV/Vis spectroscopy. Tests performed revealed high sensitivities and limits of detection equal to 10 ± 3 M-12 and 7.7 ± 0.3 M-12 for As(III) and As(V), respectively.
Keywords: arsenic pollution; enzyme; gold nanoparticles.
© 2016 The Author(s).
Figures



Similar articles
-
A New Strategy for As(V) Biosensing Based on the Inhibition of the Phosphatase Activity of the Arsenate Reductase from Thermus thermophilus.Int J Mol Sci. 2022 Mar 9;23(6):2942. doi: 10.3390/ijms23062942. Int J Mol Sci. 2022. PMID: 35328363 Free PMC article.
-
Interaction of Thermus thermophilus ArsC enzyme and gold nanoparticles naked-eye assays speciation between As(III) and As(V).Nanotechnology. 2015 Oct 30;26(43):435703. doi: 10.1088/0957-4484/26/43/435703. Epub 2015 Oct 5. Nanotechnology. 2015. Retraction in: Nanotechnology. 2023 Mar 30;34(24). doi: 10.1088/1361-6528/acc643. PMID: 26436536 Retracted.
-
A novel arsenate reductase from the bacterium Thermus thermophilus HB27: its role in arsenic detoxification.Biochim Biophys Acta. 2013 Oct;1834(10):2071-9. doi: 10.1016/j.bbapap.2013.06.007. Epub 2013 Jun 22. Biochim Biophys Acta. 2013. PMID: 23800470
-
DNAzyme-functionalized gold nanoparticles for biosensing.Adv Biochem Eng Biotechnol. 2014;140:93-120. doi: 10.1007/10_2013_242. Adv Biochem Eng Biotechnol. 2014. PMID: 24026635 Review.
-
Gold nanoparticle-based signal amplification for biosensing.Anal Biochem. 2011 Oct 1;417(1):1-16. doi: 10.1016/j.ab.2011.05.027. Epub 2011 May 26. Anal Biochem. 2011. PMID: 21703222 Review.
Cited by
-
A New Strategy for As(V) Biosensing Based on the Inhibition of the Phosphatase Activity of the Arsenate Reductase from Thermus thermophilus.Int J Mol Sci. 2022 Mar 9;23(6):2942. doi: 10.3390/ijms23062942. Int J Mol Sci. 2022. PMID: 35328363 Free PMC article.
-
Extremophiles, a Nifty Tool to Face Environmental Pollution: From Exploitation of Metabolism to Genome Engineering.Int J Environ Res Public Health. 2021 May 14;18(10):5228. doi: 10.3390/ijerph18105228. Int J Environ Res Public Health. 2021. PMID: 34069056 Free PMC article. Review.
-
Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics?Int J Mol Sci. 2019 Jun 4;20(11):2747. doi: 10.3390/ijms20112747. Int J Mol Sci. 2019. PMID: 31167476 Free PMC article. Review.
-
An ArsR/SmtB family member regulates arsenic resistance genes unusually arranged in Thermus thermophilus HB27.Microb Biotechnol. 2017 Nov;10(6):1690-1701. doi: 10.1111/1751-7915.12761. Epub 2017 Jul 11. Microb Biotechnol. 2017. PMID: 28696001 Free PMC article.
-
Genomic Insight of Alicyclobacillus mali FL18 Isolated From an Arsenic-Rich Hot Spring.Front Microbiol. 2021 Apr 8;12:639697. doi: 10.3389/fmicb.2021.639697. eCollection 2021. Front Microbiol. 2021. PMID: 33897644 Free PMC article.
References
-
- Smedley PL, Kinniburgh DG. 2002. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 17, 517–568. (10.1016/S0883-2927(02)00018-5) - DOI
-
- Moore JW, Ramamoorthy S. 2012. Heavy metals in natural waters: applied monitoring and impact assessment. Berlin, Germany: Springer Science & Business Media.
-
- Casentini B, Pettine M, Millero FJ. 2010. Release of arsenic from volcanic rocks through interactions with inorganic anions and organic ligands. Aquat. Geochem. 16, 373–393. (10.1007/s10498-010-9090-3) - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

