Glutamate Clearance Is Locally Modulated by Presynaptic Neuronal Activity in the Cerebral Cortex
- PMID: 27707974
- PMCID: PMC5050332
- DOI: 10.1523/JNEUROSCI.2066-16.2016
Glutamate Clearance Is Locally Modulated by Presynaptic Neuronal Activity in the Cerebral Cortex
Abstract
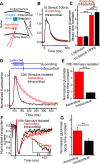
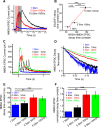
Excitatory amino acid transporters (EAATs) are abundantly expressed by astrocytes, rapidly remove glutamate from the extracellular environment, and restrict the temporal and spatial extent of glutamate signaling. Studies probing EAAT function suggest that their capacity to remove glutamate is large and does not saturate, even with substantial glutamate challenges. In contrast, we report that neuronal activity rapidly and reversibly modulates EAAT-dependent glutamate transport. To date, no physiological manipulation has shown changes in functional glutamate uptake in a nonpathological state. Using iGluSnFr-based glutamate imaging and electrophysiology in the adult mouse cortex, we show that glutamate uptake is slowed up to threefold following bursts of neuronal activity. The slowing of glutamate uptake depends on the frequency and duration of presynaptic neuronal activity but is independent of the amount of glutamate released. The modulation of glutamate uptake is brief, returning to normal within 50 ms after stimulation ceases. Interestingly, the slowing of glutamate uptake is specific to activated synapses, even within the domain of an individual astrocyte. Activity-induced slowing of glutamate uptake, and the increased persistence of glutamate in the extracellular space, is reflected by increased decay times of neuronal NR2A-mediated NMDA currents. These results show that astrocytic clearance of extracellular glutamate is slowed in a temporally and spatially specific manner following bursts of neuronal activity ≥30 Hz and that these changes affect the neuronal response to released glutamate. This suggests a previously unreported form of neuron-astrocyte interaction.
Significance statement: We report the first fast, physiological modulation of astrocyte glutamate clearance kinetics. We show that presynaptic activity in the cerebral cortex increases the persistence of glutamate in the extracellular space by slowing its clearance by astrocytes. Because of abundant EAAT expression, glutamate clearance from the extracellular space has been thought to have invariant kinetics. While multiple studies report experimental manipulations resulting in altered EAAT expression, our findings show that astrocytic glutamate uptake is dynamic on a fast time-scale. This shows rapid plasticity of glutamate clearance, which locally modulates synaptic signaling in the cortex. As astrocytic glutamate uptake is a fundamental and essential mechanism for neurotransmission, this work has implications for neurotransmission, extrasynaptic receptor activation, and synaptic plasticity.
Keywords: activity; astrocyte; cortex; glutamate; glutamate uptake.
Copyright © 2016 the authors 0270-6474/16/3610404-12$15.00/0.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
