Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism
- PMID: 27708054
- PMCID: PMC5539971
- DOI: 10.1126/science.aaf6284
Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism
Abstract
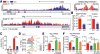
Aerobic glycolysis (the Warburg effect) is a metabolic hallmark of activated T cells and has been implicated in augmenting effector T cell responses, including expression of the proinflammatory cytokine interferon-γ (IFN-γ), via 3' untranslated region (3'UTR)-mediated mechanisms. Here, we show that lactate dehydrogenase A (LDHA) is induced in activated T cells to support aerobic glycolysis but promotes IFN-γ expression independently of its 3'UTR. Instead, LDHA maintains high concentrations of acetyl-coenzyme A to enhance histone acetylation and transcription of Ifng Ablation of LDHA in T cells protects mice from immunopathology triggered by excessive IFN-γ expression or deficiency of regulatory T cells. These findings reveal an epigenetic mechanism by which aerobic glycolysis promotes effector T cell differentiation and suggest that LDHA may be targeted therapeutically in autoinflammatory diseases.
Copyright © 2016, American Association for the Advancement of Science.
Figures
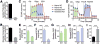


Comment in
-
Warburg meets epigenetics.Science. 2016 Oct 28;354(6311):419-420. doi: 10.1126/science.aak9776. Science. 2016. PMID: 27789830 No abstract available.
-
Metabolic Control of Cellular Differentiation.Dev Cell. 2016 Nov 7;39(3):286-287. doi: 10.1016/j.devcel.2016.10.019. Dev Cell. 2016. PMID: 27825439
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

