Rapid development of a DNA vaccine for Zika virus
- PMID: 27708058
- PMCID: PMC5304212
- DOI: 10.1126/science.aai9137
Rapid development of a DNA vaccine for Zika virus
Abstract
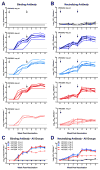
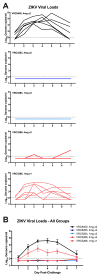
Zika virus (ZIKV) was identified as a cause of congenital disease during the explosive outbreak in the Americas and Caribbean that began in 2015. Because of the ongoing fetal risk from endemic disease and travel-related exposures, a vaccine to prevent viremia in women of childbearing age and their partners is imperative. We found that vaccination with DNA expressing the premembrane and envelope proteins of ZIKV was immunogenic in mice and nonhuman primates, and protection against viremia after ZIKV challenge correlated with serum neutralizing activity. These data not only indicate that DNA vaccination could be a successful approach to protect against ZIKV infection, but also suggest a protective threshold of vaccine-induced neutralizing activity that prevents viremia after acute infection.
Copyright © 2016, American Association for the Advancement of Science.
Figures


References
-
- Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nature reviews Microbiology. 2007;5:518–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

