Generation of an inducible colon-specific Cre enzyme mouse line for colon cancer research
- PMID: 27708166
- PMCID: PMC5081651
- DOI: 10.1073/pnas.1614057113
Generation of an inducible colon-specific Cre enzyme mouse line for colon cancer research
Abstract
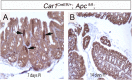
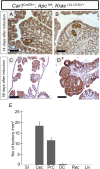
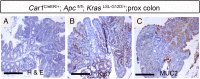
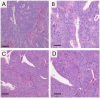
Current mouse models for colorectal cancer often differ significantly from human colon cancer, being largely restricted to the small intestine. Here, we aim to develop a colon-specific inducible mouse model that can faithfully recapitulate human colon cancer initiation and progression. Carbonic anhydrase I (Car1) is a gene expressed uniquely in colonic epithelial cells. We generated a colon-specific inducible Car1CreER knock-in (KI) mouse with broad Cre activity in epithelial cells of the proximal colon and cecum. Deletion of the tumor suppressor gene Apc using the Car1CreER KI caused tumor formation in the cecum but did not yield adenomas in the proximal colon. Mutation of both Apc and Kras yielded microadenomas in both the cecum and the proximal colon, which progressed to macroadenomas with significant morbidity. Aggressive carcinomas with some invasion into lymph nodes developed upon combined induction of oncogenic mutations of Apc, Kras, p53, and Smad4 Importantly, no adenomas were observed in the small intestine. Additionally, we observed tumors from differentiated Car1-expressing cells with Apc/Kras mutations, suggesting that a top-down model of intestinal tumorigenesis can occur with multiple mutations. Our results establish the Car1CreER KI as a valuable mouse model to study colon-specific tumorigenesis and metastasis as well as cancer-cell-of-origin questions.
Keywords: Car1; colorectal cancer; differentiated epithelial cells; gastrointestinal tract; mouse model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
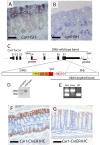
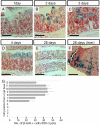
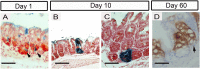
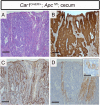



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

