T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts
- PMID: 27708227
- PMCID: PMC5363558
- DOI: 10.18632/oncotarget.12357
T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts
Abstract
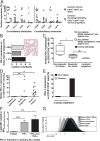
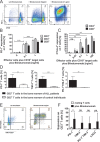
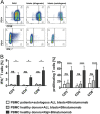
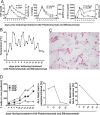
T-cell immunotherapies are promising options in relapsed/refractory B-precursor acute lymphoblastic leukemia (ALL). We investigated the effect of co-signaling molecules on T-cell attack against leukemia mediated by CD19/CD3-bispecific T-cell engager. Primary CD19+ ALL blasts (n≥10) and physiologic CD19+CD10+ bone marrow precursors were screened for 20 co-signaling molecules. PD-L1, PD-1, LAG-3, CD40, CD86, CD27, CD70 and HVEM revealed different stimulatory and inhibitory profiles of pediatric ALL compared to physiologic cells, with PD-L1 and CD86 as most prominent inhibitory and stimulatory markers. PD-L1 was increased in relapsed ALL patients (n=11) and in ALLs refractory to Blinatumomab (n=5). Exhaustion markers (PD-1, TIM-3) were significantly higher on patients' T cells compared to physiologic controls. T-cell proliferation and effector function was target-cell dependent and correlated to expression of co-signaling molecules. Blockade of inhibitory PD-1-PD-L and CTLA-4-CD80/86 pathways enhanced T-cell function whereas blockade of co-stimulatory CD28-CD80/86 interaction significantly reduced T-cell function. Combination of Blinatumomab and anti-PD-1 antibody was feasible and induced an anti-leukemic in vivo response in a 12-year-old patient with refractory ALL. In conclusion, ALL cells actively regulate T-cell function by expression of co-signaling molecules and modify efficacy of therapeutic T-cell attack against ALL. Inhibitory interactions of leukemia-induced checkpoint molecules can guide future T-cell therapies.
Keywords: CD80/86; PD-L1; T cells; blinatumomab; immune checkpoints; pediatric acute lymphoblastic leukemia.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



Similar articles
-
Extramedullary relapse and discordant CD19 expression between bone marrow and extramedullary sites in relapsed acute lymphoblastic leukemia after blinatumomab treatment.Curr Probl Cancer. 2019 Jun;43(3):222-227. doi: 10.1016/j.currproblcancer.2018.04.006. Epub 2018 May 7. Curr Probl Cancer. 2019. PMID: 29895435
-
Potential for bispecific T-cell engagers: role of blinatumomab in acute lymphoblastic leukemia.Drug Des Devel Ther. 2016 Feb 18;10:757-65. doi: 10.2147/DDDT.S83848. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 26937176 Free PMC article. Review.
-
Blinatumomab: a CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy.Leuk Lymphoma. 2016 May;57(5):1021-32. doi: 10.3109/10428194.2016.1161185. Epub 2016 Apr 6. Leuk Lymphoma. 2016. PMID: 27050240 Review.
-
Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia.J Hematol Oncol. 2015 Sep 4;8:104. doi: 10.1186/s13045-015-0195-4. J Hematol Oncol. 2015. PMID: 26337639 Free PMC article. Review.
-
Redirecting T cells to eradicate B-cell acute lymphoblastic leukemia: bispecific T-cell engagers and chimeric antigen receptors.Leukemia. 2017 Apr;31(4):777-787. doi: 10.1038/leu.2016.391. Epub 2016 Dec 28. Leukemia. 2017. PMID: 28028314 Review.
Cited by
-
Tumor Microenvironment Immunosuppression: A Roadblock to CAR T-Cell Advancement in Solid Tumors.Cells. 2022 Nov 16;11(22):3626. doi: 10.3390/cells11223626. Cells. 2022. PMID: 36429054 Free PMC article. Review.
-
The landscape of bispecific T cell engager in cancer treatment.Biomark Res. 2021 May 26;9(1):38. doi: 10.1186/s40364-021-00294-9. Biomark Res. 2021. PMID: 34039409 Free PMC article. Review.
-
New immune cell engagers for cancer immunotherapy.Nat Rev Immunol. 2024 Jul;24(7):471-486. doi: 10.1038/s41577-023-00982-7. Epub 2024 Jan 25. Nat Rev Immunol. 2024. PMID: 38273127 Review.
-
T Cell Subsets During Early Life and Their Implication in the Treatment of Childhood Acute Lymphoblastic Leukemia.Front Immunol. 2021 Mar 4;12:582539. doi: 10.3389/fimmu.2021.582539. eCollection 2021. Front Immunol. 2021. PMID: 33763058 Free PMC article. Review.
-
Exogenous signaling repairs defective T cell signaling inside the tumor microenvironment for better immunity.JCI Insight. 2022 Sep 8;7(17):e159479. doi: 10.1172/jci.insight.159479. JCI Insight. 2022. PMID: 36073543 Free PMC article.
References
-
- Handgretinger R, Zugmaier G, Henze G, Kreyenberg H, Lang P, von Stackelberg A. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia. 2011;25:181–184. - PubMed
-
- Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. The Lancet Oncology. 2013;14:e205–217. - PubMed
-
- Zugmaier G, Klinger M, Schmidt M, Subklewe M. Clinical overview of anti-CD19 BiTE and ex vivo data from anti-CD33 BiTE as examples for retargeting T cells in hematologic malignancies. Molecular Immunology. 2015;67:58–66. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

