Crystal structures of the human elongation factor eEFSec suggest a non-canonical mechanism for selenocysteine incorporation
- PMID: 27708257
- PMCID: PMC5059743
- DOI: 10.1038/ncomms12941
Crystal structures of the human elongation factor eEFSec suggest a non-canonical mechanism for selenocysteine incorporation
Abstract
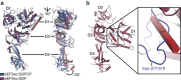
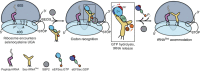
Selenocysteine is the only proteinogenic amino acid encoded by a recoded in-frame UGA codon that does not operate as the canonical opal stop codon. A specialized translation elongation factor, eEFSec in eukaryotes and SelB in prokaryotes, promotes selenocysteine incorporation into selenoproteins by a still poorly understood mechanism. Our structural and biochemical results reveal that four domains of human eEFSec fold into a chalice-like structure that has similar binding affinities for GDP, GTP and other guanine nucleotides. Surprisingly, unlike in eEF1A and EF-Tu, the guanine nucleotide exchange does not cause a major conformational change in domain 1 of eEFSec, but instead induces a swing of domain 4. We propose that eEFSec employs a non-canonical mechanism involving the distinct C-terminal domain 4 for the release of the selenocysteinyl-tRNA during decoding on the ribosome.
Figures




Similar articles
-
On elongation factor eEFSec, its role and mechanism during selenium incorporation into nascent selenoproteins.Biochim Biophys Acta Gen Subj. 2018 Nov;1862(11):2463-2472. doi: 10.1016/j.bbagen.2018.03.018. Epub 2018 Mar 17. Biochim Biophys Acta Gen Subj. 2018. PMID: 29555379 Free PMC article. Review.
-
The selenocysteine-specific elongation factor contains a novel and multi-functional domain.J Biol Chem. 2012 Nov 9;287(46):38936-45. doi: 10.1074/jbc.M112.415463. Epub 2012 Sep 19. J Biol Chem. 2012. PMID: 22992746 Free PMC article.
-
Crystal structure of the full-length bacterial selenocysteine-specific elongation factor SelB.Nucleic Acids Res. 2015 Oct 15;43(18):9028-38. doi: 10.1093/nar/gkv833. Epub 2015 Aug 24. Nucleic Acids Res. 2015. PMID: 26304550 Free PMC article.
-
Selenocysteine tRNA-specific elongation factor SelB is a structural chimaera of elongation and initiation factors.EMBO J. 2005 Jan 12;24(1):11-22. doi: 10.1038/sj.emboj.7600505. Epub 2004 Dec 23. EMBO J. 2005. PMID: 15616587 Free PMC article.
-
Ribosome dynamics during decoding.Philos Trans R Soc Lond B Biol Sci. 2017 Mar 19;372(1716):20160182. doi: 10.1098/rstb.2016.0182. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28138068 Free PMC article. Review.
Cited by
-
Ribosome Fate during Decoding of UGA-Sec Codons.Int J Mol Sci. 2021 Dec 8;22(24):13204. doi: 10.3390/ijms222413204. Int J Mol Sci. 2021. PMID: 34948001 Free PMC article. Review.
-
Association of rs142548867 (EEFSEC) and periodontitis Grade C in a young Brazilian population.J Appl Oral Sci. 2023 Jul 17;31:e20230058. doi: 10.1590/1678-7757-2023-0058. eCollection 2023. J Appl Oral Sci. 2023. PMID: 37466550 Free PMC article.
-
EEFSEC deficiency: A selenopathy with early-onset neurodegeneration.Am J Hum Genet. 2025 Jan 2;112(1):168-180. doi: 10.1016/j.ajhg.2024.12.001. Am J Hum Genet. 2025. PMID: 39753114 Free PMC article.
-
On elongation factor eEFSec, its role and mechanism during selenium incorporation into nascent selenoproteins.Biochim Biophys Acta Gen Subj. 2018 Nov;1862(11):2463-2472. doi: 10.1016/j.bbagen.2018.03.018. Epub 2018 Mar 17. Biochim Biophys Acta Gen Subj. 2018. PMID: 29555379 Free PMC article. Review.
-
A Common Genetic Factor Underlies Genetic Risk for Gynaecological and Reproductive Disorders and Is Correlated with Risk to Depression.Neuroendocrinology. 2023;113(10):1059-1075. doi: 10.1159/000533413. Epub 2023 Aug 4. Neuroendocrinology. 2023. PMID: 37544299 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

