Obesity-associated NLRC4 inflammasome activation drives breast cancer progression
- PMID: 27708283
- PMCID: PMC5059727
- DOI: 10.1038/ncomms13007
Obesity-associated NLRC4 inflammasome activation drives breast cancer progression
Abstract
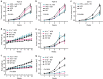
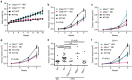
Obesity is associated with an increased risk of developing breast cancer and is also associated with worse clinical prognosis. The mechanistic link between obesity and breast cancer progression remains unclear, and there has been no development of specific treatments to improve the outcome of obese cancer patients. Here we show that obesity-associated NLRC4 inflammasome activation/ interleukin (IL)-1 signalling promotes breast cancer progression. The tumour microenvironment in the context of obesity induces an increase in tumour-infiltrating myeloid cells with an activated NLRC4 inflammasome that in turn activates IL-1β, which drives disease progression through adipocyte-mediated vascular endothelial growth factor A (VEGFA) expression and angiogenesis. Further studies show that treatment of mice with metformin inhibits obesity-associated tumour progression associated with a marked decrease in angiogenesis. This report provides a causal mechanism by which obesity promotes breast cancer progression and lays out a foundation to block NLRC4 inflammasome activation or IL-1β signalling transduction that may be useful for the treatment of obese cancer patients.
Figures





References
-
- Flegal K. M., Kit B. K. & Graubard B. I. Overweight, obesity, and all-cause mortality--reply. JAMA 309, 1681–1682 (2013). - PubMed
-
- Rose D. P. & Vona-Davis L. Influence of obesity on breast cancer receptor status and prognosis. Expert Rev. Anticancer Ther. 9, 1091–1101 (2009). - PubMed
-
- Reeves G. K., Pirie K., Green J., Bull D. & Beral V. Comparison of the effects of genetic and environmental risk factors on in situ and invasive ductal breast cancer. Int. J. Cancer 131, 930–937 (2012). - PubMed
-
- Gilbert C. A. & Slingerland J. M. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu. Rev. Med. 64, 45–57 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 CA158055/CA/NCI NIH HHS/United States
- R00 CA158055/CA/NCI NIH HHS/United States
- T32 AI007260/AI/NIAID NIH HHS/United States
- K01 DK111758/DK/NIDDK NIH HHS/United States
- T32 AI007485/AI/NIAID NIH HHS/United States
- T32 AI007517/AI/NIAID NIH HHS/United States
- P30 CA086862/CA/NCI NIH HHS/United States
- K22 CA118182/CA/NCI NIH HHS/United States
- L60 MD006355/MD/NIMHD NIH HHS/United States
- L30 CA199587/CA/NCI NIH HHS/United States
- R01 CA200673/CA/NCI NIH HHS/United States
- R56 AI118719/AI/NIAID NIH HHS/United States
- R01 AI104706/AI/NIAID NIH HHS/United States
- R01 CA203834/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

