Role of areca nut induced JNK/ATF2/Jun axis in the activation of TGF-β pathway in precancerous Oral Submucous Fibrosis
- PMID: 27708346
- PMCID: PMC5052620
- DOI: 10.1038/srep34314
Role of areca nut induced JNK/ATF2/Jun axis in the activation of TGF-β pathway in precancerous Oral Submucous Fibrosis
Abstract
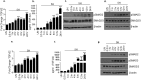
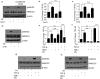
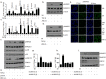
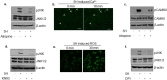
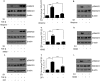
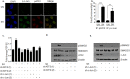
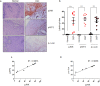
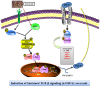
Oral submucous fibrosis (OSF) is potentially premalignant with progressive and irreversible extracellular matrix deposition accompanied by epithelial atrophy and like other fibrotic disorders, is primarily a TGF-β driven disease. OSF is caused by prolonged chewing of areca nut. Our previous studies reported a pivotal role for TGF-β activation and its effects contributing to OSF. However, the mechanism for activation of TGF-β signaling in OSF is still unknown. In this study we demonstrate activation of TGF-β signaling with sub-cytotoxic dose of areca nut in epithelial cells and discovered a key role for pJNK in this process. In good correlation; pJNK was detected in OSF tissues but not in normal tissues. Moreover, activation of JNK was found to be dependent on muscarinic acid receptor induced Ca2+/CAMKII as well as ROS. JNK dependent phosphorylation of ATF2/c-Jun transcription factors resulted in TGF-β transcription and its signaling. pATF2/p-c-Jun were enriched on TGF-β promoter and co-localized in nuclei of epithelial cells upon areca nut treatment. In corroboration, OSF tissue sections also had nuclear pATF2 and p-c-Jun. Our results provide comprehensive mechanistic details of TGF-β signaling induced by etiological agent areca nut in the manifestation of fibrosis which can lead to new therapeutic modalities for OSF.
Figures


References
-
- IARC. Tobacco habits other than smoking; betel quid and areca-nut chewing; and some related nitrosamines. IARC Working Group. Lyon, 23–30 October 1984. IARC Monogr Eval Carcinog Risk Chem to Hum 37, 1–268 (1985). - PubMed
-
- Lee C. H. et al.. Intercountry prevalences and practices of betel-quid use in south, southeast and eastern Asia regions and associated oral preneoplastic disorders: an international collaborative study by Asian betel-quid consortium of south and east Asia. Int J Cancer 129, 1741–1751 (2011). - PubMed
-
- Chu N. S. Effects of Betel chewing on the central and autonomic nervous systems. J Biomed Sci 8, 229–236 (2001). - PubMed
-
- Bouchner B. J. & Mannan N. Metabolic effects of the consumption of Areca catechu. Addict Biol 7, 103–110 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

