Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress
- PMID: 27708387
- PMCID: PMC5052518
- DOI: 10.1038/srep34768
Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress
Abstract
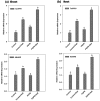
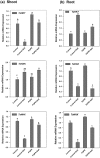
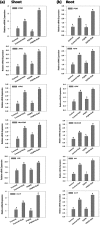
Plant growth promoting rhizobacteria (PGPR) hold promising future for sustainable agriculture. Here, we demonstrate a carotenoid producing halotolerant PGPR Dietzia natronolimnaea STR1 protecting wheat plants from salt stress by modulating the transcriptional machinery responsible for salinity tolerance in plants. The expression studies confirmed the involvement of ABA-signalling cascade, as TaABARE and TaOPR1 were upregulated in PGPR inoculated plants leading to induction of TaMYB and TaWRKY expression followed by stimulation of expression of a plethora of stress related genes. Enhanced expression of TaST, a salt stress-induced gene, associated with promoting salinity tolerance was observed in PGPR inoculated plants in comparison to uninoculated control plants. Expression of SOS pathway related genes (SOS1 and SOS4) was modulated in PGPR-applied wheat shoots and root systems. Tissue-specific responses of ion transporters TaNHX1, TaHAK, and TaHKT1, were observed in PGPR-inoculated plants. The enhanced gene expression of various antioxidant enzymes such as APX, MnSOD, CAT, POD, GPX and GR and higher proline content in PGPR-inoculated wheat plants contributed to increased tolerance to salinity stress. Overall, these results indicate that halotolerant PGPR-mediated salinity tolerance is a complex phenomenon that involves modulation of ABA-signalling, SOS pathway, ion transporters and antioxidant machinery.
Figures





References
-
- Huang G. T. et al.. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 39, 969–987 (2012). - PubMed
-
- Agarwal P. K. & Jha B. Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Biol. Plantarum. 54, 201–212 (2010).
-
- Rajendran K., Tester M. & Roy S. J. Quantifying the three main components of salinity tolerance in cereals. Plant Cell Environ. 32, 237–249 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

