Adherence and Patients' Experiences with the Use of Capecitabine in Daily Practice
- PMID: 27708578
- PMCID: PMC5030243
- DOI: 10.3389/fphar.2016.00310
Adherence and Patients' Experiences with the Use of Capecitabine in Daily Practice
Abstract
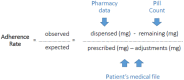
Introduction: Capecitabine is a widely prescribed oral anticancer agent. We studied medication adherence and explored its use in daily practice from a patients' perspective. Patients and Methods: Patients (n = 92) starting capecitabine were followed up to five 3-week cycles. Adherence was assessed using a pill count, pharmacy data and dosing information from the patients' medical file. Self-reported adherence was measured using the Medication Adherence Report Scale (MARS). At baseline and during week 2 of cycles 1, 3, and 5, patients filled out questionnaires about quality of life, symptoms, attitude toward medicines and disease and use in daily practice. Simultaneously, blood samples were taken to determine the area under the curve (AUC) of 5'-deoxy-5-fluorouridine (5'-DFUR), 5-fluorouracil (5-FU), and α-fluoro-β-alanine (FBAL) by a population pharmacokinetic model. Associations between AUCs and patient-reported symptoms were tested for cycles 3 and 5. Results: Most patients (84/92; 91%) had an adherence rate of ≥95 and ≤ 105%. The percentage of patients reporting any non-adherence behavior measured with MARS increased from 16% at cycle 1 to 29% at cycle 5. Symptoms were reported frequently and the dosing regimen was adjusted by the physician at least once in 62% of patients. In multivariate analysis the probability of an adjustment increased with the number of co-medication (OR 1.19, 95% CI: 1.03-1.39) and a stronger emotional response to the disease (OR 1.32, 95% CI: 1.10-1.59). The AUC of 5'-DFUR was associated with weight loss (OR 1.10, 95% CI: 1.01-1.19), AUC of FBAL with hand-foot syndrome (OR 0.90, 95% CI: 0.83-0.99), rhinorrhea (OR 1.21, 95% CI: 1.03-1.42 weight loss (OR 1.09, 95% CI: 1.00-1.20) and depression (OR 0.90, 95% CI: 0.82-0.99). Side effects were reported by one third of patients as the reason to discontinue treatment. Conclusion: Adherence to capecitabine was generally high. Nevertheless, adherence measured with MARS decreased over time Adherence management to support implementation of correct capecitabine use is specifically relevant in longer term treatment. In addition, it appears that adverse event management is important to support persistence. With the extending armamentarium of oral targeted anticancer agents and prolonged treatment duration, we expect the issue of medication adherence of increasing importance in oncology.
Keywords: capecitabine; dose adjustments; medication adherence; oral anticancer agent; patient-reported symptoms; pharmacokinetics.
Figures
References
-
- Basch E., Reeve B. B., Mitchell S. A., Clauser S. B., Minasian L. M., Dueck A. C., et al. . (2014). Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J. Natl. Cancer Inst. 106:dju244. 10.1093/jnci/dju244 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous