Preparation and Characterization of Loperamide-Loaded Dynasan 114 Solid Lipid Nanoparticles for Increased Oral Absorption In the Treatment of Diarrhea
- PMID: 27708583
- PMCID: PMC5030211
- DOI: 10.3389/fphar.2016.00332
Preparation and Characterization of Loperamide-Loaded Dynasan 114 Solid Lipid Nanoparticles for Increased Oral Absorption In the Treatment of Diarrhea
Abstract
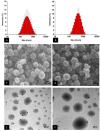
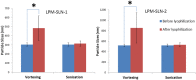
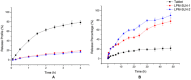
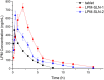
The aim of the project was to assemble two optimum solid lipid nanoparticle (SLN) formulations for oral delivery of loperamide (LPM) to treat different types of diarrhea, and to evaluate their release profiles in vitro and pharmacokinetic properties in vivo. In this work, glyceryl trimyristate (Dynasan 114) nanoparticles containing the drug LPM and sodium cholate as a stabilizer were prepared using a modified solvent evaporation technique. Two LPM-loaded SLNs, namely LPM-SLN-1 (LPM-SLN with a high ratio rate of lipid to drug) and LPM-SLN-2 (LPM-SLN with a low ratio rate of lipid to drug), were prepared by the solvent evaporation method. A change in the lipid concentration affects the characteristics of LPM-SLNs. The average sizes of the LPM-SLNs were 303 ± 18 nm and 519 ± 36 nm, separately, as analyzed by dynamic light scattering. The LPM-SLNs were found to be round with a smooth surface, as observed using a transmission electron microscope and a scanning electron microscope. The average encapsulation efficiencies were 87 ± 3.78% w/w and 84 ± 5.17%, accordingly. In the in vitro release experiments, LPM-SLNs showed a continuous release profile of LPM without any burst release. The oral bioavailability of LPM-SLNs was analyzed using Wistar rats. The relative bioavailabilities of LPM-SLNs were 227 and 153%, respectively, as compared that of the LPM tablet. There was no difference in the Tmax between LPM-SLN-2 and the LPM tablet. In conclusion, LPM-SLN-1 significantly improved the oral bioavailability of LPM, while LPM-SLN-2 having the same swift action as the LPM tablet. These results demonstrate the potential of LPM-SLNs in the oral delivery of LPM to treat different types of diarrhea.
Keywords: loperamide; oral delivery formulation; poorly water-soluble drugs; solid lipid nanoparticles; sustained release.
Figures

Similar articles
-
Naringenin-loaded solid lipid nanoparticles: preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics.Drug Des Devel Ther. 2016 Mar 1;10:911-25. doi: 10.2147/DDDT.S97738. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27041995 Free PMC article.
-
Development of Domperidone Solid Lipid Nanoparticles: In Vitro and In Vivo Characterization.AAPS PharmSciTech. 2018 May;19(4):1712-1719. doi: 10.1208/s12249-018-0987-2. Epub 2018 Mar 12. AAPS PharmSciTech. 2018. PMID: 29532427
-
Pharmacokinetic and pharmacodynamic studies of nisoldipine-loaded solid lipid nanoparticles developed by central composite design.Drug Dev Ind Pharm. 2015;41(12):1968-77. doi: 10.3109/03639045.2015.1024685. Epub 2015 Apr 1. Drug Dev Ind Pharm. 2015. PMID: 25830370
-
Development of repaglinide loaded solid lipid nanocarrier: selection of fabrication method.Curr Drug Deliv. 2010 Jan;7(1):44-50. doi: 10.2174/156720110790396472. Curr Drug Deliv. 2010. PMID: 20044909 Review.
-
Solid lipid nanoparticles, an effective carrier for classical antifungal drugs.Saudi Pharm J. 2023 Jul;31(7):1167-1180. doi: 10.1016/j.jsps.2023.05.011. Epub 2023 May 19. Saudi Pharm J. 2023. PMID: 37273269 Free PMC article. Review.
Cited by
-
Characterization of lipid-based nanomedicines at the single-particle level.Fundam Res. 2022 Oct 3;3(4):488-504. doi: 10.1016/j.fmre.2022.09.011. eCollection 2023 Jul. Fundam Res. 2022. PMID: 38933557 Free PMC article. Review.
-
Metal ion-responsive nanocarrier derived from phosphonated calix[4]arenes for delivering dauricine specifically to sites of brain injury in a mouse model of intracerebral hemorrhage.J Nanobiotechnology. 2020 Apr 19;18(1):61. doi: 10.1186/s12951-020-00616-3. J Nanobiotechnology. 2020. PMID: 32306970 Free PMC article.
-
Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: formulation and clinical study.Drug Deliv. 2018 Nov;25(1):78-90. doi: 10.1080/10717544.2017.1413444. Drug Deliv. 2018. PMID: 29239242 Free PMC article. Clinical Trial.
-
Development of Perphenazine-Loaded Solid Lipid Nanoparticles: Statistical Optimization and Cytotoxicity Studies.Biomed Res Int. 2021 Apr 28;2021:6619195. doi: 10.1155/2021/6619195. eCollection 2021. Biomed Res Int. 2021. PMID: 33997026 Free PMC article.
-
Using pyrene to probe the effects of poloxamer stabilisers on internal lipid microenvironments in solid lipid nanoparticles.Nanoscale Adv. 2020 Oct 19;2(12):5572-5577. doi: 10.1039/d0na00582g. eCollection 2020 Dec 15. Nanoscale Adv. 2020. PMID: 36133871 Free PMC article.
References
-
- Abdelwahed W. (2015). Lyophilization of solid lipid nanoparticles for brain targeting. Int. J. Pharm. Pharm. Sci. 7 10.1016/B978-0-12-391860-4.00012-4 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

