In situ, Reversible Gating of a Mechanosensitive Ion Channel through Protein-Lipid Interactions
- PMID: 27708587
- PMCID: PMC5030285
- DOI: 10.3389/fphys.2016.00409
In situ, Reversible Gating of a Mechanosensitive Ion Channel through Protein-Lipid Interactions
Abstract
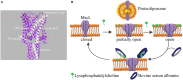
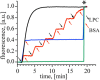
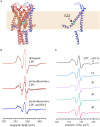
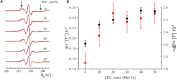
Understanding the functioning of ion channels, as well as utilizing their properties for biochemical applications requires control over channel activity. Herein we report a reversible control over the functioning of a mechanosensitive ion channel by interfering with its interaction with the lipid bilayer. The mechanosensitive channel of large conductance from Escherichia coli is reconstituted into liposomes and activated to its different sub-open states by titrating lysophosphatidylcholine (LPC) into the lipid bilayer. Activated channels are closed back by the removal of LPC out of the membrane by bovine serum albumin (BSA). Electron paramagnetic resonance spectra showed the LPC-dose-dependent gradual opening of the channel pore in the form of incrementally increasing spin label mobility and decreasing spin-spin interaction. A method to reversibly open and close mechanosensitive channels to distinct sub-open conformations during their journey from the closed to the fully open state enables detailed structural studies to follow the conformational changes during channel functioning. The ability of BSA to revert the action of LPC opens new perspectives for the functional studies of other membrane proteins that are known to be activated by LPC.
Keywords: MscL; bovine serum albumin; electron spin resonance spectroscopy; lysophosphatidylcholines; mechanosensation; reversible gating.
Figures
Similar articles
-
The activation mode of the mechanosensitive ion channel, MscL, by lysophosphatidylcholine differs from tension-induced gating.FASEB J. 2014 Oct;28(10):4292-302. doi: 10.1096/fj.14-251579. Epub 2014 Jun 23. FASEB J. 2014. PMID: 24958207 Free PMC article.
-
Inducible release of particulates from liposomes using the mechanosensitive channel of large conductance and L-α-lysophosphatidylcholine.Eur Biophys J. 2015 Oct;44(7):521-30. doi: 10.1007/s00249-015-1055-4. Epub 2015 Jul 5. Eur Biophys J. 2015. PMID: 26143502
-
Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating.Nat Struct Biol. 2002 Sep;9(9):696-703. doi: 10.1038/nsb827. Nat Struct Biol. 2002. PMID: 12172537
-
Mechanosensitive channels in bacteria as membrane tension reporters.FASEB J. 1999;13 Suppl:S55-61. doi: 10.1096/fasebj.13.9001.s55. FASEB J. 1999. PMID: 10352145 Review.
-
Approaches for the modulation of mechanosensitive MscL channel pores.Front Chem. 2023 Mar 15;11:1162412. doi: 10.3389/fchem.2023.1162412. eCollection 2023. Front Chem. 2023. PMID: 37021145 Free PMC article. Review.
Cited by
-
Protein Conformational Dynamics upon Association with the Surfaces of Lipid Membranes and Engineered Nanoparticles: Insights from Electron Paramagnetic Resonance Spectroscopy.Molecules. 2020 Nov 18;25(22):5393. doi: 10.3390/molecules25225393. Molecules. 2020. PMID: 33218036 Free PMC article. Review.
-
Tuning ion channel mechanosensitivity by asymmetry of the transbilayer pressure profile.Biophys Rev. 2018 Oct;10(5):1377-1384. doi: 10.1007/s12551-018-0450-3. Epub 2018 Sep 4. Biophys Rev. 2018. PMID: 30182202 Free PMC article. Review.
-
Activating mechanosensitive channels embedded in droplet interface bilayers using membrane asymmetry.Chem Sci. 2021 Jan 4;12(6):2138-2145. doi: 10.1039/d0sc03889j. Chem Sci. 2021. PMID: 34163978 Free PMC article.
References
-
- Blount P., Iscla I., Moe P. C., Li Y. (2007). MscL: the bacterial mechanosensitive channel of large conductance, in Current Topics in Membranes, Vol. 58, ed Hamill O. P. (Texas: Elsevier; ), 201–233.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

