The Bone Marrow-Derived Stromal Cells: Commitment and Regulation of Adipogenesis
- PMID: 27708616
- PMCID: PMC5030474
- DOI: 10.3389/fendo.2016.00127
The Bone Marrow-Derived Stromal Cells: Commitment and Regulation of Adipogenesis
Abstract
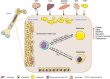
Bone marrow (BM) microenvironment represents an important compartment of bone that regulates bone homeostasis and the balance between bone formation and bone resorption depending on the physiological needs of the organism. Abnormalities of BM microenvironmental dynamics can lead to metabolic bone diseases. BM stromal cells (also known as skeletal or mesenchymal stem cells) [bone marrow stromal stem cell (BMSC)] are multipotent stem cells located within BM stroma and give rise to osteoblasts and adipocytes. However, cellular and molecular mechanisms of BMSC lineage commitment to adipocytic lineage and regulation of BM adipocyte formation are not fully understood. In this review, we will discuss recent findings pertaining to identification and characterization of adipocyte progenitor cells in BM and the regulation of differentiation into mature adipocytes. We have also emphasized the clinical relevance of these findings.
Keywords: adipogenesis; bone marrow microenvironment; bone marrow stem cell subpopulations; bone marrow stem cells; secreted factors.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

