Construction of Efficient 3D Gas Evolution Electrocatalyst for Hydrogen Evolution: Porous FeP Nanowire Arrays on Graphene Sheets
- PMID: 27709000
- PMCID: PMC5032976
- DOI: 10.1002/advs.201500120
Construction of Efficient 3D Gas Evolution Electrocatalyst for Hydrogen Evolution: Porous FeP Nanowire Arrays on Graphene Sheets
Abstract
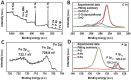
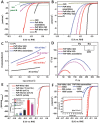
A novel 3D hierarchical nanocomposite of vertically aligned porous FeP nano-wires on reduced graphene oxide is prepared as a demonstration of constructing an efficient hydrogen evolution catalyst. Extension of this nanostructuring strategy to other functional nanocomposites by combining different dimensional nanomaterials is attractive.
Keywords: hierarchical nanocomposites; hydrogen evolution reaction; porous FeP nanowire arrays; pseudomorphic transformation.
Figures



References
-
- a) Laursen A. B., Kegnaes S., Dahl S., Chorkendorff I., Energy Environ. Sci. 2012, 5, 5577;
- b) Benck J. D., Hellstern T. R., Kibsgaard J., Chakthranont P., Jaramillo T. F., ACS Catal. 2014, 4, 3957;
- c) Tan C., Zhang H., Chem. Soc. Rev. 2014, 43, 4281; - PubMed
- d) Yan Y., Xia B., Xu Z., Wang X., ACS Catal. 2014, 4, 1693;
- e) Li G., Zhang B., Yu F., Novakova A. A., Krivenkov M. S., Kiseleva T. Y., Chang L., Rao J., Polyakov A. O., Blake G. R., de Groot R. A., Palstra T. T. M., Chem. Mater. 2014, 26, 5821.
-
- a) Xie J., Li S., Zhang X., Zhang J., Wang R., Zhang H., Pan B., Xie Y., Chem. Sci. 2014, 5, 4615;
- b) Chen W.‐F., Sasaki K., Ma C., Frenkel A. I., Marinkovic N., Muckerman J. T., Zhu Y., Adzic R. R., Angew. Chem. 2012, 124, 6235; - PubMed
- c) Chen W. F., Wang C. H., Sasaki K., Marinkovic N., Xu W., Muckerman J. T., Zhu Y., Adzic R. R., Energy Environ. Sci. 2013, 6, 943;
- d) Lai X., Halpert J. E., Wang D., Energy Environ. Sci. 2012, 5, 5604;
- e) Duan J., Chen S., Jaroniec M., Qiao S. Z., ACS Nano 2015, 9, 931. - PubMed
-
- a) Chen W. F., Muckerman J. T., Fujita E., Chem. Commun. 2013, 49, 8896; - PubMed
- b) Wan C., Regmi Y. N., Leonard B. M., Angew. Chem. Int. Ed. 2014, 53, 6407; - PubMed
- c) Cao B., Veith G. M., Neuefeind J. C., Adzic R. R., Khalifah P. G., J. Am. Chem. Soc. 2013, 135, 19186; - PubMed
- d) Zhang H., Wang Y., Wang D., Li Y., Liu X., Liu P., Yang H., An T., Tang Z., Zhao H., Small 2014, 10, 3371. - PubMed
-
- a) Xing Z., Liu Q., Asiri A. M., Sun X., Adv. Mater. 2014, 26, 5702; - PubMed
- b) Huang Z., Chen Z., Chen Z., Lv C., Meng H., Zhang C., ACS Nano 2014, 8, 8121; - PubMed
- c) McEnaney J. M., Crompton J. C., Callejas J. F., Popczun E. J., Biacchi A. J., Lewis N. S., Schaak R. E., Chem. Mater. 2014, 26, 4826;
- d) Pu Z., Liu Q., Jiang P., Asiri A. M., Obaid A. Y., Sun X., Chem. Mater. 2014, 26, 4326;
- e) Jiang P., Liu Q., Sun X., Nanoscale 2014, 6, 13440; - PubMed
- f) Bai Y., Zhang H., Li X., Liu L., Xu H., Qiu H., Wang Y., Nanoscale 2015, 7, 1446; - PubMed
- g) Pu Z., Liu Q., Tang C., Asiri A. M., Sun X., Nanoscale 2014, 6, 11031; - PubMed
- h) Popczun E. J., Read C. G., Roske C. W., Lewis N. S., Schaak R. E., Angew. Chem. Int. Ed. 2014, 53, 5427; - PubMed
- i) Xing Z., Liu Q., Asiri A. M., Sun X., ACS Catal. 2015, 5, 145;
- j) Xiao P., Sk M. A., Thia L., Ge X., Lim R. J., Wang J.‐Y., Lim K. H., Wang X., Energy Environ. Sci. 2014, 7, 2624;
- k) Oyama S. T., J. Catal. 2003, 216, 343;
- l) Zheng Y., Jiao Y., Jaroniec M., Qiao S. Z., Angew. Chem. Int. Ed. 2015, 54, 52. - PubMed
-
- Oyama S. T., Gott T., Zhao H., Lee Y.‐K., Catal. Today 2009, 143, 94.
LinkOut - more resources
Full Text Sources
Other Literature Sources
