Effect of age on the pharmacokinetics of polymorphic nimodipine in rats after oral administration
- PMID: 27709016
- PMCID: PMC5045546
- DOI: 10.1016/j.apsb.2016.07.010
Effect of age on the pharmacokinetics of polymorphic nimodipine in rats after oral administration
Abstract
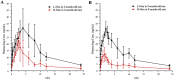
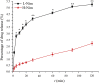
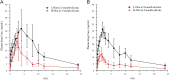
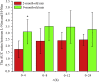
The previous investigation has proved that their existed pharmacokinetic difference between the different crystal forms of the polymorphic drugs after oral administration. However, no systemic investigations have been made on the change of this pharmacokinetic difference, resulted either from the physiological or from the pathological factors. In this paper, we used polymorphic nimodipine (Nim) as a model drug and investigated the effect of age difference (2- and 9-month old) on the pharmacokinetics after oral delivery in rats. As the results shown, for L-form of Nim (L-Nim), the AUC0-24 h in 2-month-old rats was 343.68±47.15 ng·h/mL, which is 23.36% higher than that in 9-month-old rats. For H-form of Nim (H-Nim), the AUC0-24 h in 2-month-old rats was 140.91±19.47 ng·h/mL, which is 54.64% higher than that in 9-month-old rats. The AUC0-24 h ratio between H-Nim and L-Nim was 2.44 in 2-month-old rats and 3.06 in 9-month-old rats. Since age difference could result in unparallelled change of the absorption and bioavailability of the polymorphic drugs, the results in this experiment are of value for further investigation of crystal form selection in clinical trials and rational clinical application of the polymorphic drugs.
Keywords: Age difference; Crystal form; Nimodipine; Pharmacokinetics; Polymorphic drug.
Figures

References
-
- Xu C.H., Zou M.J., Liu Y., Ren J.G., Tian Y., Yan J. Pharmacokinetics of carbamazepine polymorphs and dihydrate in rats, related to dogs and humans. Arch Pharm Res. 2011;34:1973–1982. - PubMed
-
- Sun Z.L., Yao D., Yuan J.P., Yuan G.Y., Bu F.L., Jiang Z.M. Human pharmacokinetic variations of different nitrendipine crystalline polymorphs. Lat Am J Pharm. 2014;33:1182–1187.
-
- Chinsangaram J., Honeychurch K.M., Tyavanagimatt S.R., Bolken T.C., Jordan R., Jones K.F. Pharmacokinetic comparison of a single oral dose of polymorph form I versus form V capsules of the antiorthopoxvirus compound ST-246 in human volunteers. Antimicrob Agents Chemother. 2012;56:3582–3586. - PMC - PubMed
-
- Wang H.R., Zhang L.T., Wang Q., Yuan Z.F., Li M. Validated LC–MS–MS method for determination of m-nisoldipine polymorphs in rat plasma and its application to pharmacokinetic studies. J Chromatogr B. 2006;835:71–76. - PubMed
-
- Du W., Zhou Y.F., Gong Y.F., Zhao C.S. Investigation of physicochemical properties and in-vitro in-vivo evaluation of agomelatine polymorphs. Asian J Pharm Sci. 2013;8:181–190.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

