EGF-Amphiregulin Interplay in Airway Stem/Progenitor Cells Links the Pathogenesis of Smoking-Induced Lesions in the Human Airway Epithelium
- PMID: 27709733
- PMCID: PMC5330845
- DOI: 10.1002/stem.2512
EGF-Amphiregulin Interplay in Airway Stem/Progenitor Cells Links the Pathogenesis of Smoking-Induced Lesions in the Human Airway Epithelium
Abstract
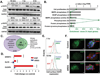
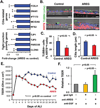
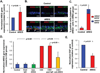
The airway epithelium of cigarette smokers undergoes dramatic remodeling with hyperplasia of basal cells (BC) and mucus-producing cells, squamous metaplasia, altered ciliated cell differentiation and decreased junctional barrier integrity, relevant to chronic obstructive pulmonary disease and lung cancer. In this study, we show that epidermal growth factor receptor (EGFR) ligand amphiregulin (AREG) is induced by smoking in human airway epithelium as a result of epidermal growth factor (EGF)-driven squamous differentiation of airway BC stem/progenitor cells. In turn, AREG induced a unique EGFR activation pattern in human airway BC, distinct from that evoked by EGF, leading to BC- and mucous hyperplasia, altered ciliated cell differentiation and impaired barrier integrity. Further, AREG promoted its own expression and suppressed expression of EGF, establishing an autonomous self-amplifying signaling loop in airway BC relevant for promotion of EGF-independent hyperplastic phenotypes. Thus, EGF-AREG interplay in airway BC stem/progenitor cells is one of the mechanisms that mediates the interconnected pathogenesis of all major smoking-induced lesions in the human airway epithelium. Stem Cells 2017;35:824-837.
Keywords: Airway basal cells; Chronic obstructive pulmonary disease; Epidermal growth factor receptor; Hyperplasia; Metaplasia.
© 2016 AlphaMed Press.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

