Randomized Trial of Aromatase Inhibitors, Growth Hormone, or Combination in Pubertal Boys with Idiopathic, Short Stature
- PMID: 27710241
- PMCID: PMC5155684
- DOI: 10.1210/jc.2016-2891
Randomized Trial of Aromatase Inhibitors, Growth Hormone, or Combination in Pubertal Boys with Idiopathic, Short Stature
Abstract
Context: Growth of short children in puberty is limited by the effect of estrogen on epiphyseal fusion.
Objectives: To compare: 1) the efficacy and safety of aromatase inhibitors (AIs) vs GH vs AI/GH on increasing adult height potential in pubertal boys with severe idiopathic short stature (ISS); and 2) differences in body composition among groups.
Design: Randomized three-arm open-label comparator.
Setting: Outpatient clinical research.
Patients: Seventy-six pubertal boys [mean (SE) age, 14.1 (0.1) years] with ISS [height SD score (SDS), -2.3 (0.0)].
Intervention: Daily AIs (anastrozole or letrozole), GH, or AI/GH for 24-36 months.
Outcomes: Anthropometry, bone ages, dual x-ray absorptiometry, spine x-rays, hormones, safety labs.
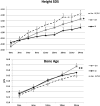
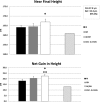
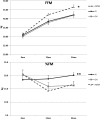
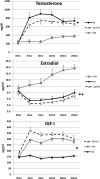
Results: Height gain [mean (SE)] at 24 months was: AI, +14.0 (0.8) cm; GH, +17.1 (0.9) cm; AI/GH, +18.9 (0.8) cm (P < .0006, analysis of covariance). Height SDS was: AI, -1.73 (0.12); GH, -1.43 (0.14); AI/GH, -1.25 (0.12) (P < .0012). Those treated through 36 months grew more. Regardless of treatment duration, height SDS at near-final height [n = 71; age, 17.4 (0.2) years; bone age, 15.3 (0.1) years; height achieved, ∼97.6%] was: AI, -1.4 (0.1); GH, -1.4 (0.2); AI/GH, -1.0 (0.1) (P = .06). Absolute height change was: AI, +18.2 (1.6) cm; GH, +20.6 (1.5) cm; AI/GH, +22.5 (1.4) cm (P = .01) (expected height gain at -2.0 height SDS, +13.0 cm). AI/GH had higher fat free mass accrual. Measures of bone health, safety labs, and adverse events were similar in all groups. Letrozole caused higher T and lower estradiol than anastrozole.
Conclusions: Combination therapy with AI/GH increases height potential in pubertal boys with ISS more than GH and AI alone treated for 24-36 months with a strong safety profile.
Figures
References
-
- Mauras N, Attie KM, Reiter EO, Saenger P, Baptista J. High dose recombinant human growth hormone (GH) treatment of GH-deficient patients in puberty increases near-final height: a randomized, multicenter trial. Genentech, Inc., Cooperative Study Group. J Clin Endocrinol Metab. 2000;85(10):3653–3660. - PubMed
-
- Mericq MV, Eggers M, Avila A, Cutler GB, Jr, Cassorla F. Near final height in pubertal growth hormone (GH)-deficient patients treated with GH alone or in combination with luteinizing hormone-releasing hormone analog: results of a prospective, randomized trial. J Clin Endocrinol Metab. 2000;85(2):569–573. - PubMed
-
- Pucarelli I, Segni M, Ortore M, Arcadi E, Pasquino AM. Effects of combined gonadotropin-releasing hormone agonist and growth hormone therapy on adult height in precocious puberty: a further contribution. J Pediatr Endocrinol Metab. 2003;16(7):1005–1010. - PubMed
-
- Reiter EO. A brief review of the addition of gonadotropin-releasing hormone agonists (GnRH-Ag) to growth hormone (GH) treatment of children with idiopathic growth hormone deficiency: previously published studies from America. Mol Cell Endocrinol. 2006;254–255:221–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

