RADVAN: a randomised phase 2 trial of WBRT plus vandetanib for melanoma brain metastases - results and lessons learnt
- PMID: 27711083
- PMCID: PMC5104891
- DOI: 10.1038/bjc.2016.318
RADVAN: a randomised phase 2 trial of WBRT plus vandetanib for melanoma brain metastases - results and lessons learnt
Abstract
Background: Brain metastases occur in up to 75% of patients with advanced melanoma. Most are treated with whole-brain radiotherapy (WBRT), with limited effectiveness. Vandetanib, an inhibitor of vascular endothelial growth factor receptor, epidermal growth factor receptor and rearranged during transfection tyrosine kinases, is a potent radiosensitiser in xenograft models. We compared WBRT with WBRT plus vandetanib in the treatment of patients with melanoma brain metastases.
Methods: In this double-blind, multi-centre, phase 2 trial patients with melanoma brain metastases were randomised to receive WBRT (30 Gy in 10 fractions) plus 3 weeks of concurrent vandetanib 100 mg once daily or placebo. The primary endpoint was progression-free survival in brain (PFS brain). The main study was preceded by a safety run-in phase to confirm tolerability of the combination. A post-hoc analysis and literature review considered barriers to recruiting patients with melanoma brain metastases to clinical trials.
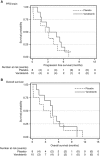
Results: Twenty-four patients were recruited, six to the safety phase and 18 to the randomised phase. The study closed early due to poor recruitment. Median PFS brain was 3.3 months (90% confidence interval (CI): 1.6-5.6) in the vandetanib group and 2.5 months (90% CI: 0.2-4.8) in the placebo group (P=0.34). Median overall survival (OS) was 4.6 months (90% CI: 1.6-6.3) and 2.5 months (90% CI: 0.2-7.2), respectively (P=0.54). The most frequent adverse events were fatigue, alopecia, confusion and nausea. The most common barrier to study recruitment was availability of alternative treatments.
Conclusions: The combination of WBRT plus vandetanib was well tolerated. Compared with WBRT alone, there was no significant improvement in PFS brain or OS, although we are unable to provide a definitive result due to poor accrual. A review of barriers to trial accrual identified several factors that affect study recruitment in this difficult disease area.
Conflict of interest statement
MRM has consulted for and received research funding from AstraZeneca, GlaxoSmithKline and Roche. All other authors have declared no conflicts of interest.
Figures
Similar articles
-
Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial.Lancet Oncol. 2012 Sep;13(9):897-905. doi: 10.1016/S1470-2045(12)70335-2. Epub 2012 Aug 14. Lancet Oncol. 2012. PMID: 22898678 Clinical Trial.
-
Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial.Lancet Oncol. 2010 Jul;11(7):619-26. doi: 10.1016/S1470-2045(10)70132-7. Lancet Oncol. 2010. PMID: 20570559 Free PMC article. Clinical Trial.
-
Randomized trial of erlotinib plus whole-brain radiotherapy for NSCLC patients with multiple brain metastases.J Natl Cancer Inst. 2014 Jul 16;106(7):dju151. doi: 10.1093/jnci/dju151. Print 2014 Jul. J Natl Cancer Inst. 2014. PMID: 25031274 Free PMC article. Clinical Trial.
-
Surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis.Cochrane Database Syst Rev. 2018 Aug 20;8(8):CD012086. doi: 10.1002/14651858.CD012086.pub2. Cochrane Database Syst Rev. 2018. PMID: 30125049 Free PMC article.
-
Vandetanib: in medullary thyroid cancer.Drugs. 2012 Jul 9;72(10):1423-36. doi: 10.2165/11209300-000000000-00000. Drugs. 2012. PMID: 22715896 Review.
Cited by
-
Cardiovascular Toxicities with Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors in Cancer Patients: A Meta-Analysis of 77 Randomized Controlled Trials.Clin Drug Investig. 2018 Dec;38(12):1109-1123. doi: 10.1007/s40261-018-0709-2. Clin Drug Investig. 2018. PMID: 30327999 Review.
-
Risk of gastrointestinal events with newly approved (after 2011) vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a meta-analysis of randomized controlled trials.Eur J Clin Pharmacol. 2017 Oct;73(10):1209-1217. doi: 10.1007/s00228-017-2299-y. Epub 2017 Jul 15. Eur J Clin Pharmacol. 2017. PMID: 28710508
-
Radiotherapy for Melanoma: More than DNA Damage.Dermatol Res Pract. 2019 Apr 3;2019:9435389. doi: 10.1155/2019/9435389. eCollection 2019. Dermatol Res Pract. 2019. PMID: 31073304 Free PMC article. Review.
-
Systematic literature review and meta-analysis of clinical outcomes and prognostic factors for melanoma brain metastases.Front Oncol. 2022 Dec 8;12:1025664. doi: 10.3389/fonc.2022.1025664. eCollection 2022. Front Oncol. 2022. PMID: 36568199 Free PMC article.
-
The impact of current treatment modalities on the outcomes of patients with melanoma brain metastases: A systematic review.Int J Cancer. 2020 Mar 15;146(6):1479-1489. doi: 10.1002/ijc.32696. Epub 2019 Nov 23. Int J Cancer. 2020. PMID: 31583684 Free PMC article.
References
-
- Agarwala SS, Kirkwood JM, Gore M, Dreno B, Thatcher N, Czarnetski B, Atkins M, Buzaid A, Skarlos D, Rankin EM (2004) Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol 22: 2101–2107. - PubMed
-
- Ajithkumar T, Parkinson C, Fife K, Corrie P, Jefferies S (2015) Evolving treatment options for melanoma brain metastases. Lancet Oncol 16: e486–e497. - PubMed
-
- Atkins MB, Sosman JA, Agarwala S, Logan T, Clark JI, Ernstoff MS, Lawson D, Dutcher JP, Weiss G, Curti B, Margolin KA (2008) Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: a phase II Cytokine Working Group study. Cancer 113: 2139–2145. - PubMed
-
- Bible KC, Ryder M (2016) Evolving molecularly targeted therapies for advanced-stage thyroid cancers. Nat Rev Clin Oncol 13(7): 403–416. - PubMed
-
- Chen G, Chakravarti N, Aardalen K, Lazar AJ, Tetzlaff MT, Wubbenhorst B, Kim SB, Kopetz S, Ledoux AA, Gopal YN, Pereira CG, Deng W, Lee JS, Nathanson KL, Aldape KD, Prieto VG, Stuart D, Davies MA (2014) Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the PI3K pathway as a therapeutic target. Clin Cancer Res 20: 5537–5546. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials