Altered Modulation of Silent Period in Tongue Motor Cortex of Persistent Developmental Stuttering in Relation to Stuttering Severity
- PMID: 27711148
- PMCID: PMC5053488
- DOI: 10.1371/journal.pone.0163959
Altered Modulation of Silent Period in Tongue Motor Cortex of Persistent Developmental Stuttering in Relation to Stuttering Severity
Abstract
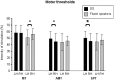
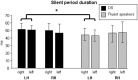
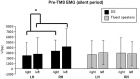
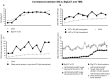
Motor balance in developmental stuttering (DS) was investigated with Transcranial Magnetic Stimulation (TMS), with the aim to define novel neural markers of persistent DS in adulthood. Eleven DS adult males were evaluated with TMS on tongue primary motor cortex, compared to 15 matched fluent speakers, in a "state" condition (i.e. stutterers vs. fluent speakers, no overt stuttering). Motor and silent period thresholds (SPT), recruitment curves, and silent period durations were acquired by recording tongue motor evoked potentials. Tongue silent period duration was increased in DS, especially in the left hemisphere (P<0.05; Hedge's g or Cohen's dunbiased = 1.054, i.e. large effect size), suggesting a "state" condition of higher intracortical inhibition in left motor cortex networks. Differences in motor thresholds (different excitatory/inhibitory ratios in DS) were evident, as well as significant differences in SPT. In fluent speakers, the left hemisphere may be marginally more excitable than the right one in motor thresholds at lower muscular activation, while active motor thresholds and SPT were higher in the left hemisphere of DS with respect to the right one, resulting also in a positive correlation with stuttering severity. Pre-TMS electromyography data gave overlapping evidence. Findings suggest the existence of a complex intracortical balance in DS tongue primary motor cortex, with a particular interplay between excitatory and inhibitory mechanisms, also in neural substrates related to silent periods. Findings are discussed with respect to functional and structural impairments in stuttering, and are also proposed as novel neural markers of a stuttering "state" in persistent DS, helping to define more focused treatments (e.g. neuro-modulation).
Conflict of interest statement
ABC Balbuzie is a commercial affiliation. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Speech dynamics are coded in the left motor cortex in fluent speakers but not in adults who stutter.Brain. 2015 Mar;138(Pt 3):712-25. doi: 10.1093/brain/awu390. Epub 2015 Jan 15. Brain. 2015. PMID: 25595146 Free PMC article.
-
Motor excitability evaluation in developmental stuttering: a transcranial magnetic stimulation study.Cortex. 2013 Mar;49(3):781-92. doi: 10.1016/j.cortex.2011.12.002. Epub 2011 Dec 16. Cortex. 2013. PMID: 22225881
-
Stuttering as a matter of delay in neural activation: A combined TMS/EEG study.Clin Neurophysiol. 2019 Jan;130(1):61-76. doi: 10.1016/j.clinph.2018.10.005. Epub 2018 Nov 10. Clin Neurophysiol. 2019. PMID: 30476712
-
Transcranial magnetic stimulation in developmental stuttering: Relations with previous neurophysiological research and future perspectives.Clin Neurophysiol. 2017 Jun;128(6):952-964. doi: 10.1016/j.clinph.2017.03.039. Epub 2017 Apr 3. Clin Neurophysiol. 2017. PMID: 28431323 Review.
-
Developmental stuttering and the role of the supplementary motor cortex.J Fluency Disord. 2020 Jun;64:105763. doi: 10.1016/j.jfludis.2020.105763. Epub 2020 Apr 20. J Fluency Disord. 2020. PMID: 32361030 Review.
Cited by
-
Speech Fluency Improvement in Developmental Stuttering Using Non-invasive Brain Stimulation: Insights From Available Evidence.Front Hum Neurosci. 2021 Aug 11;15:662016. doi: 10.3389/fnhum.2021.662016. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34456692 Free PMC article. Review.
-
Biomarkers Obtained by Transcranial Magnetic Stimulation in Neurodevelopmental Disorders.J Clin Neurophysiol. 2022 Feb 1;39(2):135-148. doi: 10.1097/WNP.0000000000000784. J Clin Neurophysiol. 2022. PMID: 34366399 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

