Cell-Free Phospholipid Biosynthesis by Gene-Encoded Enzymes Reconstituted in Liposomes
- PMID: 27711229
- PMCID: PMC5053487
- DOI: 10.1371/journal.pone.0163058
Cell-Free Phospholipid Biosynthesis by Gene-Encoded Enzymes Reconstituted in Liposomes
Abstract
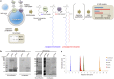
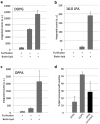
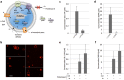
The goal of bottom-up synthetic biology culminates in the assembly of an entire cell from separate biological building blocks. One major challenge resides in the in vitro production and implementation of complex genetic and metabolic pathways that can support essential cellular functions. Here, we show that phospholipid biosynthesis, a multiple-step process involved in cell membrane homeostasis, can be reconstituted starting from the genes encoding for all necessary proteins. A total of eight E. coli enzymes for acyl transfer and headgroup modifications were produced in a cell-free gene expression system and were co-translationally reconstituted in liposomes. Acyl-coenzyme A and glycerol-3-phosphate were used as canonical precursors to generate a variety of important bacterial lipids. Moreover, this study demonstrates that two-step acyl transfer can occur from enzymes synthesized inside vesicles. Besides clear implications for growth and potentially division of a synthetic cell, we postulate that gene-based lipid biosynthesis can become instrumental for ex vivo and protein purification-free production of natural and non-natural lipids.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

