The Role of Hepatitis C Virus Core Antigen Testing in the Era of Direct Acting Antiviral Therapies: What We Can Learn from the Protease Inhibitors
- PMID: 27711230
- PMCID: PMC5053597
- DOI: 10.1371/journal.pone.0163900
The Role of Hepatitis C Virus Core Antigen Testing in the Era of Direct Acting Antiviral Therapies: What We Can Learn from the Protease Inhibitors
Abstract
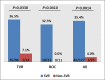
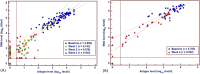
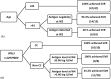
Direct-acting antiviral (DAA) therapies have revolutionised the treatment of hepatitis C virus (HCV). The financial cost of DAAs however is significant, and first generation protease inhibitors (PIs) also require frequent monitoring of viral RNA levels to guide treatment. In this context, we examined the relevance of HCV antigen testing to evaluate the potential role in monitoring virological response to HCV antiviral treatment with the PI-based triple therapies, telaprevir (TVR) and boceprevir (BOC). Chronic HCV-infected individuals (n = 152) enrolled in the Irish Hepatitis C Outcomes Research Network (ICORN) study were prospectively analysed for baseline markers and the early viral kinetics associated with SVR. The sustained virological response (SVR) rates in the cohort receiving TVR and BOC were 87.3% and 73.8%, respectively. Baseline factors associated with successful outcome in TVR therapy were age (P = 0.0098), IFNL3 genotype (P = 0.0330) and viral load (P = 0.0456). RNA level at week 4 (P = 0.0068) and viral antigen negativity at week 2 (P = 0.0359) were predictive of SVR for TVR-based therapy. In BOC therapy, prior interferon treatment (P = 0.0209) and IFNL3 genotype (P = 0.0410) were baseline predictors of SVR. Evidence of viraemia based either on viral RNA or antigen at week 4 predicted SVR in these patients. Our data showed that rapid decline of HCV antigen to negative level at week 2 in TVR treatment and <0.96 log fmol/l in BOC treatment after commencement of PI triple therapy were associated with SVR. HCV antigen measurement should be considered as a potential alternative for monitoring treatment response during DAA-based regimens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
HCV viral load at baseline and at week 4 of telaprevir/boceprevir based triple therapies are associated with virological outcome in HIV/hepatitis C co-infected patients.J Clin Virol. 2015 Dec;73:32-35. doi: 10.1016/j.jcv.2015.10.010. Epub 2015 Oct 19. J Clin Virol. 2015. PMID: 26528903
-
Early virologic responses and hematologic safety of direct-acting antiviral therapies in veterans with chronic hepatitis C.Clin Gastroenterol Hepatol. 2013 Aug;11(8):1021-7. doi: 10.1016/j.cgh.2013.03.006. Epub 2013 Mar 21. Clin Gastroenterol Hepatol. 2013. PMID: 23524130
-
Protease inhibitor-based triple therapy is highly effective for hepatitis C recurrence after liver transplant: a multicenter experience.Ann Hepatol. 2014 Sep-Oct;13(5):525-32. Ann Hepatol. 2014. PMID: 25152985
-
Therapy of chronic hepatitis C virus infection in the era of direct-acting and host-targeting antiviral agents.J Infect. 2014 Jan;68(1):1-20. doi: 10.1016/j.jinf.2013.08.019. Epub 2013 Sep 4. J Infect. 2014. PMID: 24012819 Review.
-
Telaprevir: changing the standard of care of chronic hepatitis C.J Postgrad Med. 2013 Jan-Mar;59(1):42-7. doi: 10.4103/0022-3859.109493. J Postgrad Med. 2013. PMID: 23525057 Review.
Cited by
-
Hepatitis C Core Antigen Testing: Still an Effective Diagnostic Method for Global Elimination of Hepatitis C.Clin Infect Dis. 2020 Feb 3;70(4):674-675. doi: 10.1093/cid/ciz273. Clin Infect Dis. 2020. PMID: 30943285 Free PMC article. No abstract available.
-
HCV core antigen is an alternative marker to HCV RNA for evaluating active HCV infection: implications for improved diagnostic option in an era of affordable DAAs.PeerJ. 2017 Nov 6;5:e4008. doi: 10.7717/peerj.4008. eCollection 2017. PeerJ. 2017. PMID: 29134150 Free PMC article.
-
Clinical utility of hepatitis C virus core antigen assay in the monitoring of direct-acting antivirals for chronic hepatitis C.PLoS One. 2020 Mar 3;15(3):e0229994. doi: 10.1371/journal.pone.0229994. eCollection 2020. PLoS One. 2020. PMID: 32126125 Free PMC article.
-
Hepatitis C Virus-Core Antigen: Implications in Diagnostic, Treatment Monitoring and Clinical Outcomes.Viruses. 2024 Nov 29;16(12):1863. doi: 10.3390/v16121863. Viruses. 2024. PMID: 39772172 Free PMC article. Review.
-
HCV core antigen plays an important role in the fight against HCV as an alternative to HCV-RNA detection.J Clin Lab Anal. 2021 Jun;35(6):e23755. doi: 10.1002/jcla.23755. Epub 2021 Mar 31. J Clin Lab Anal. 2021. PMID: 33788295 Free PMC article. Review.
References
-
- Morota K, Fujinami R, Kinukawa H, Machida T, Ohno K, Saegusa H, et al. A new sensitive and automated chemiluminescent microparticle immunoassay for quantitative determination of hepatitis C virus core antigen. Journal of virological methods. 2009;157(1):8–14. Epub 2009/01/13. 10.1016/j.jviromet.2008.12.009 . - DOI - PubMed
-
- Loggi E, Cursaro C, Scuteri A, Grandini E, Panno AM, Galli S, et al. Patterns of HCV-RNA and HCV core antigen in the early monitoring of standard treatment for chronic hepatitis C. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2013;56(3):207–11. Epub 2012/12/19. 10.1016/j.jcv.2012.11.012 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

