The Effect of Gender on Mesenchymal Stem Cell (MSC) Efficacy in Neonatal Hyperoxia-Induced Lung Injury
- PMID: 27711256
- PMCID: PMC5053475
- DOI: 10.1371/journal.pone.0164269
The Effect of Gender on Mesenchymal Stem Cell (MSC) Efficacy in Neonatal Hyperoxia-Induced Lung Injury
Abstract
Background: Mesenchymal stem cells (MSC) improve alveolar and vascular structures in experimental models of bronchopulmonary dysplasia (BPD). Female MSC secrete more anti-inflammatory and pro-angiogenic factors as compared to male MSC. Whether the therapeutic efficacy of MSC in attenuating lung injury in an experimental model of BPD is influenced by the sex of the donor MSC or recipient is unknown. Here we tested the hypothesis that female MSC would have greater lung regenerative properties than male MSC in experimental BPD and this benefit would be more evident in males.
Objective: To determine whether intra-tracheal (IT) administration of female MSC to neonatal rats with experimental BPD has more beneficial reparative effects as compared to IT male MSC.
Methods: Newborn Sprague-Dawley rats exposed to normoxia (RA) or hyperoxia (85% O2) from postnatal day (P) 2- P21 were randomly assigned to receive male or female IT bone marrow (BM)-derived green fluorescent protein (GFP+) MSC (1 x 106 cells/50 μl), or Placebo on P7. Pulmonary hypertension (PH), vascular remodeling, alveolarization, and angiogenesis were assessed at P21. PH was determined by measuring right ventricular systolic pressure (RVSP) and pulmonary vascular remodeling was evaluated by quantifying the percentage of muscularized peripheral pulmonary vessels. Alveolarization was evaluated by measuring mean linear intercept (MLI) and radial alveolar count (RAC). Angiogenesis was determined by measuring vascular density. Data are expressed as mean ± SD, and analyzed by ANOVA.
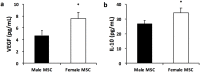
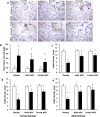
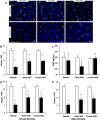
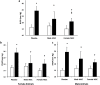
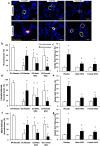
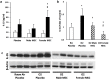
Results: There were no significant differences in the RA groups. Exposure to hyperoxia resulted in a decrease in vascular density and RAC, with a significant increase in MLI, RVSP, and the percentage of partially and fully muscularized pulmonary arterioles. Administration of both male and female MSC significantly improved vascular density, alveolarization, RVSP, percent of muscularized vessels and alveolarization. Interestingly, the improvement in PH and vascular remodeling was more robust in the hyperoxic rodents who received MSC from female donors. In keeping with our hypothesis, male animals receiving female MSC, had a greater improvement in vascular remodeling. This was accompanied by a more significant decrease in lung pro-inflammatory markers and a larger increase in anti-inflammatory and pro-angiogenic markers in male rodents that received female MSC. There were no significant differences in MSC engraftment among groups.
Conclusions: Female BM-derived MSC have greater therapeutic efficacy than male MSC in reducing neonatal hyperoxia-induced lung inflammation and vascular remodeling. Furthermore, the beneficial effects of female MSC were more pronounced in male animals. Together, these findings suggest that female MSC maybe the most potent BM-derived MSC population for lung repair in severe BPD complicated by PH.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Bhandari A, Bhandari V. “New” Bronchopulmonary Dysplasia: A Clinical Review. Clin Pulm Med. 2011;18(3):137–43 10.1097/CPM.0b013e318218a071 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

