Anti-Vascular Endothelial Growth Factor Comparative Effectiveness Trial for Diabetic Macular Edema: Additional Efficacy Post Hoc Analyses of a Randomized Clinical Trial
- PMID: 27711918
- PMCID: PMC5567802
- DOI: 10.1001/jamaophthalmol.2016.3698
Anti-Vascular Endothelial Growth Factor Comparative Effectiveness Trial for Diabetic Macular Edema: Additional Efficacy Post Hoc Analyses of a Randomized Clinical Trial
Abstract
Importance: Post hoc analyses from the Diabetic Retinopathy Clinical Research Network randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab for diabetic macular edema (DME) might influence interpretation of study results.
Objective: To provide additional outcomes comparing 3 anti-vascular endothelial growth factor (VEGF) agents for DME.
Design, setting, and participants: Post hoc analyses performed from May 3, 2016, to June 21, 2016, of a randomized clinical trial performed from August 22, 2012, to September 23, 2015, of 660 participants comparing 3 anti-VEGF treatments in eyes with center-involved DME causing vision impairment.
Exposures: Randomization to intravitreous aflibercept (2.0 mg), bevacizumab (1.25 mg), or ranibizumab (0.3 mg) administered up to monthly based on a structured retreatment regimen. Focal/grid laser treatment was added after 6 months for the treatment of persistent DME.
Main outcomes and measures: Change in visual acuity (VA) area under the curve and change in central subfield thickness (CST) within subgroups based on whether an eye received laser treatment for DME during the study.
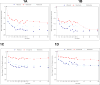
Results: Post hoc analyses were performed for 660 participants (mean [SD] age, 61 [10] years; 47% female, 65% white, 16% black or African American, 16% Hispanic, and 3% other). For eyes with an initial VA of 20/50 or worse, VA improvement was greater with aflibercept than the other agents at 1 year but superior only to bevacizumab at 2 years. Mean (SD) letter change in VA over 2 years (area under curve) was greater with aflibercept (+17.1 [9.7]) than with bevacizumab (+12.1 [9.4]; 95% CI, +1.6 to +7.3; P < .001) or ranibizumab (+13.6 [8.5]; 95% CI, +0.7 to +6.0; P = .009). When VA was 20/50 or worse at baseline, bevacizumab reduced CST less than the other agents at 1 year, but at 2 years the differences had diminished. In subgroups stratified by baseline VA, anti-VEGF agent, and whether focal/grid laser treatment was performed for DME, the only participants to have a substantial reduction in mean CST between 1 and 2 years were those with a baseline VA of 20/50 or worse receiving bevacizumab and laser treatment (mean [SD], -55 [108] µm; 95% CI, -82 to -28 µm; P < .001).
Conclusions and relevance: Although post hoc analyses should be viewed with caution given the potential for bias, in eyes with a VA of 20/50 or worse, aflibercept has the greatest improvement in VA over 2 years. Focal/grid laser treatment, ceiling and floor effects, or both may account for mean thickness reductions noted only in bevacizumab-treated eyes between 1 and 2 years.
Trial registration: clinicaltrials.gov Identifier NCT01627249.
Conflict of interest statement
John A. Wells, MD: Regeneron, Genentech (Clinical or lab research grants)
Adam R. Glassman, MS: National Institutes of Health (Grant); Genentech/Roche (Grant); Regeneron (Grant)
Allison R. Ayala: National Institutes of Health (Grant); Genentech/Roche (Grant); Regeneron (Grant)
Lee M. Jampol, MD: Janssen/QLT (Data Monitoring)
Neil M. Bressler, MD: Northwestern University (subcontract from National Eye Institute) Genentech/Roche, Lumenis, Bayer, Novartis, Regeneron (Clinical or lab research grants)
Figures
Similar articles
-
Association of Baseline Visual Acuity and Retinal Thickness With 1-Year Efficacy of Aflibercept, Bevacizumab, and Ranibizumab for Diabetic Macular Edema.JAMA Ophthalmol. 2016 Feb;134(2):127-34. doi: 10.1001/jamaophthalmol.2015.4599. JAMA Ophthalmol. 2016. PMID: 26605836 Free PMC article. Clinical Trial.
-
Persistent Macular Thickening Following Intravitreous Aflibercept, Bevacizumab, or Ranibizumab for Central-Involved Diabetic Macular Edema With Vision Impairment: A Secondary Analysis of a Randomized Clinical Trial.JAMA Ophthalmol. 2018 Mar 1;136(3):257-269. doi: 10.1001/jamaophthalmol.2017.6565. JAMA Ophthalmol. 2018. PMID: 29392288 Free PMC article. Clinical Trial.
-
Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial.Ophthalmology. 2016 Jun;123(6):1351-9. doi: 10.1016/j.ophtha.2016.02.022. Epub 2016 Feb 27. Ophthalmology. 2016. PMID: 26935357 Free PMC article. Clinical Trial.
-
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis.Cochrane Database Syst Rev. 2018 Oct 16;10(10):CD007419. doi: 10.1002/14651858.CD007419.pub6. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Jun 27;2023(6):CD007419. doi: 10.1002/14651858.CD007419.pub7. PMID: 30325017 Free PMC article. Updated.
-
Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion.Cochrane Database Syst Rev. 2020 Jul 7;7(7):CD009510. doi: 10.1002/14651858.CD009510.pub3. Cochrane Database Syst Rev. 2020. PMID: 32633861 Free PMC article.
Cited by
-
VEGFR1 signaling in retinal angiogenesis and microinflammation.Prog Retin Eye Res. 2021 Sep;84:100954. doi: 10.1016/j.preteyeres.2021.100954. Epub 2021 Feb 25. Prog Retin Eye Res. 2021. PMID: 33640465 Free PMC article. Review.
-
Intravitreal Therapy for Diabetic Macular Edema: An Update.J Ophthalmol. 2021 Feb 23;2021:6654168. doi: 10.1155/2021/6654168. eCollection 2021. J Ophthalmol. 2021. PMID: 33688431 Free PMC article. Review.
-
Factors Influencing Treatment Preference in Patients with Diabetic Macular Edema: A Study Using Conjoint Analysis.Ophthalmol Ther. 2024 Nov;13(11):2887-2901. doi: 10.1007/s40123-024-01026-6. Epub 2024 Sep 17. Ophthalmol Ther. 2024. PMID: 39287765 Free PMC article.
-
Diabetic macular edema treated with intravitreal aflibercept injection after treatment with other anti-VEGF agents (SWAP-TWO study): 6-month interim analysis.Int J Retina Vitreous. 2019 Jul 23;5:17. doi: 10.1186/s40942-019-0167-x. eCollection 2019. Int J Retina Vitreous. 2019. PMID: 31367468 Free PMC article.
-
Factors Affecting Compliance to Anti-Vascular Endothelial Growth Factor Treatment of Diabetic Macular Edema in a Cohort of Jordanian Patients.Clin Ophthalmol. 2020 Mar 24;14:921-929. doi: 10.2147/OPTH.S248661. eCollection 2020. Clin Ophthalmol. 2020. PMID: 32273676 Free PMC article.
References
-
- Ross E, Hutton D, Stein J, et al. Cost-effectiveness of Aflibercept, Bevacizumab, and Ranibizumab for Diabetic Macular Edema Treatment: Analysis from the Diabetic Retinopathy Clinical Research Network Compartative Effectiveness Trial. JAMA Ophthalmol. 2016 Jun 9; doi: 10.1001/jamaophthalmol.2016.1669. Epub ahead of print. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

