NMR Hyperpolarization Techniques of Gases
- PMID: 27711999
- PMCID: PMC5462469
- DOI: 10.1002/chem.201603884
NMR Hyperpolarization Techniques of Gases
Abstract
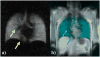
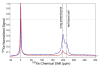
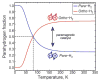
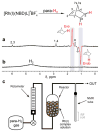
Nuclear spin polarization can be significantly increased through the process of hyperpolarization, leading to an increase in the sensitivity of nuclear magnetic resonance (NMR) experiments by 4-8 orders of magnitude. Hyperpolarized gases, unlike liquids and solids, can often be readily separated and purified from the compounds used to mediate the hyperpolarization processes. These pure hyperpolarized gases enabled many novel MRI applications including the visualization of void spaces, imaging of lung function, and remote detection. Additionally, hyperpolarized gases can be dissolved in liquids and can be used as sensitive molecular probes and reporters. This Minireview covers the fundamentals of the preparation of hyperpolarized gases and focuses on selected applications of interest to biomedicine and materials science.
Keywords: MRI; NMR; Xe-129; gas; hyperpolarization; propane.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures







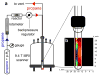



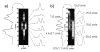

References
-
- Nikolaou P, Coffey A, Barlow M, Rosen M, Goodson B, Chekmenev E. Anal Chem. 2014;86:8206–8212. - PMC - PubMed
- Witte C, Schröder L. NMR Biomed. 2013;26:788–802. - PubMed
- Ardenkjaer-Larsen J, Fridlund B, Gram A, Hansson G, Hansson L, Lerche M, Servin R, Thaning M, Golman K. Proc Natl Acad Sci USA. 2003;100:10158–10163. - PMC - PubMed
-
- Goodson BM, Whiting N, Coffey AM, Nikolaou P, Shi F, Gust B, Gemeinhardt ME, Shchepin RV, Skinner JG, Birchall JR, Barlow MJ, Chekmenev EY. In: Hyperpolarization Methods for MRS. Griffiths J, Bottomley P, Wasylishen RE, editors. Vol. 4. John Wiley & Sons, Ltd; West Sussex, UK: 2015. pp. 797–810.
- Nikolaou P, Goodson B, Chekmenev E. Chem Eur J. 2015;21:3156–3166. - PMC - PubMed
- Goodson B. J Magn Reson. 2002;155:157–216. - PubMed
-
- Hirsch ML, Kalechofsky N, Belzer A, Rosay M, Kempf JG. J Am Chem Soc. 2015;137:8428–8434. - PubMed
-
- Walker T, Happer W. Rev Mod Phys. 1997;69:629.
-
- Ward HR. Acc Chem Res. 1972;5:18–24.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

