Redox-Neutral Aromatization of Cyclic Amines: Mechanistic Insights and Harnessing of Reactive Intermediates for Amine α- and β-C-H Functionalization
- PMID: 27712000
- PMCID: PMC5193247
- DOI: 10.1002/chem.201603839
Redox-Neutral Aromatization of Cyclic Amines: Mechanistic Insights and Harnessing of Reactive Intermediates for Amine α- and β-C-H Functionalization
Abstract
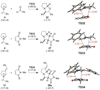
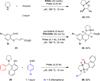
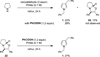
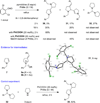
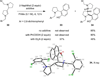
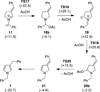
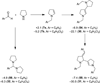
Cyclic amines such as pyrrolidine and piperidine are known to undergo condensations with aldehydes to furnish pyrrole and pyridine derivatives, respectively. A combined experimental and computational study provides detailed insights into the mechanism of pyrrole formation. A number of reactive intermediates (e.g., azomethine ylides, conjugated azomethine ylides, enamines) were intercepted, outlining strategies for circumventing aromatization as a valuable pathway for amine C-H functionalization.
Keywords: C−H functionalization; azomethine ylides; density functional theory; heterocycles; redox-neutral.
© 2016 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

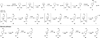








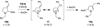

References
-
-
Pyridines from piperidine: Rügheimer L. Ber. 1891;24:2186. Rügheimer L. Ber. 1892;25:2421. Poirier RH, Morin RD, McKim AM, Bearse AE. J. Org. Chem. 1961;26:4275. Burrows EP, Hutton RF, Burrows WD. J. Org. Chem. 1962;27:316. Burrows WD, Burrows EP. J. Org. Chem. 1963;28:1180. Kameswari U, Pillai CN. Catal. Lett. 1996;38:53. Kameswari U. React. Kinet. Catal. Lett. 1996;59:135. Moura NMM, Nunez C, Santos SM, Faustino MAF, Cavaleiro JAS, Paz FAA, Neves M, Capelo JL, Lodeiro C. Chem. Eur. J. 2014;20:6684. Platonova AY, Seidel D. Tetrahedron Lett. 2015;56:3147.
-
-
-
Isoquinolines from 1,2,3,4-tetrahydroisoquinoline: Dyke SF, Sainsbury M. Tetrahedron. 1967;23:3161. Sainsbury M, Dyke SF, Brown DW, Lugton WGD. Tetrahedron. 1968;24:427. Dannhardt G, Mayer KK, Obergrusberger I, Roelcke J. Arch. Pharm. (Weinheim, Ger.) 1986;319:977. Dannhardt G, Roelcke J. Chemiker Zeitung. 1991;115:229. Dannhardt G, Roelcke J. Arch. Pharm. (Weinheim, Ger.) 1992;325:671. See also references [1e] and [1i]
-
-
-
Pyrroles from pyrrolines: Lee Y, Ling KQ, Lu X, Silverman RB, Shepard EM, Dooley DM, Sayre LM. J. Am. Chem. Soc. 2002;124:12135. Cook AG, Switek KA, Cutler KA, Witt AN. Lett. Org. Chem. 2004;1:1. Pahadi NK, Paley M, Jana R, Waetzig SR, Tunge JA. J. Am. Chem. Soc. 2009;131:16626. Xue X, Yu A, Cai Y, Cheng J-P. Org. Lett. 2011;13:6054. Fujiwara Y, Oda M, Shoji T, Abe T, Kuroda S. Heterocycles. 2012;85:1187.
-
-
-
Pyrroles from pyrrolidine: Oda M, Fukuchi Y, Ito S, Thanh NC, Kuroda S. Tetrahedron Lett. 2007;48:9159. Polackova V, Veverkova E, Toma S, Bogdal D. Synth. Commun. 2009;39:1871.
-
-
-
Indoles from indoline: Mao H, Xu R, Wan J, Jiang Z, Sun C, Pan Y. Chem. Eur. J. 2010;16:13352. Deb I, Das D, Seidel D. Org. Lett. 2011;13:812. Ramakumar K, Tunge JA. Chem. Commun. 2014;50:13056.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

