The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease
- PMID: 27713092
- PMCID: PMC6020045
- DOI: 10.1016/j.bbmt.2016.09.023
The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease
Abstract
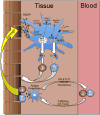
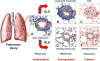
Chronic graft-versus-host disease (GVHD) is the leading cause of late, nonrelapse mortality and disability in allogeneic hematopoietic cell transplantation recipients and a major obstacle to improving outcomes. The biology of chronic GVHD remains enigmatic, but understanding the underpinnings of the immunologic mechanisms responsible for the initiation and progression of disease is fundamental to developing effective prevention and treatment strategies. The goals of this task force review are as follows: This document is intended as a review of our understanding of chronic GVHD biology and therapies resulting from preclinical studies, and as a platform for developing innovative clinical strategies to prevent and treat chronic GVHD.
Keywords: Blood and marrow transplantation; Chronic graft-versus-host disease; Clinical manifestations; Immune mechanisms.
Published by Elsevier Inc.
Figures




References
-
- Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21(2):266–74. Epub 2014/12/03. - PMC - PubMed
-
- Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–14. Epub 1998/09/25. - PubMed
-
- Kennedy GA, Varelias A, Vuckovic S, Le Texier L, Gartlan KH, Zhang P, et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2015;15(13):1451–9. Epub 2014/12/03. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

