Chronic Exposure to Low Doses of Dioxin Promotes Liver Fibrosis Development in the C57BL/6J Diet-Induced Obesity Mouse Model
- PMID: 27713108
- PMCID: PMC5332187
- DOI: 10.1289/EHP316
Chronic Exposure to Low Doses of Dioxin Promotes Liver Fibrosis Development in the C57BL/6J Diet-Induced Obesity Mouse Model
Abstract
Background: Exposure to persistent organic pollutants (POPs) has been associated with the progression of chronic liver diseases, yet the contribution of POPs to the development of fibrosis in non-alcoholic fatty liver disease (NAFLD), a condition closely linked to obesity, remains poorly documented.
Objectives: We investigated the effects of subchronic exposure to low doses of the POP 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), an aryl hydrocarbon receptor ligand, on NAFLD progression in diet-induced obese C57BL/6J mice.
Methods: Male C57BL/6J mice were fed either a 10% low-fat (LFD) or a 45% high-fat (HFD) purified diet for 14 weeks and TCDD-exposed groups were injected once a week with 5 μg/kg TCDD or the vehicle for the last 6 weeks of the diet.
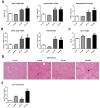
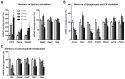
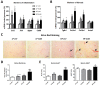
Results: Liver histology and triglyceride levels showed that exposure of HFD fed mice to TCDD worsened hepatic steatosis, as compared to either HFD alone or LFD plus TCDD and the mRNA levels of key genes of hepatic lipid metabolism were strongly altered in co-treated mice. Further, increased liver collagen staining and serum transaminase levels showed that TCDD induced liver fibrosis in the HFD fed mice. TCDD in LFD fed mice increased the expression of several inflammation and fibrosis marker genes with no additional effect from a HFD.
Conclusions: Exposure to TCDD amplifies the impairment of liver functions observed in mice fed an enriched fat diet as compared to a low fat diet. The results provide new evidence that environmental pollutants promote the development of liver fibrosis in obesity-related NAFLD in C57BL/6J mice. Citation: Duval C, Teixeira-Clerc F, Leblanc AF, Touch S, Emond C, Guerre-Millo M, Lotersztajn S, Barouki R, Aggerbeck M, Coumoul X. 2017. Chronic exposure to low doses of dioxin promotes liver fibrosis development in the C57BL/6J diet-induced obesity mouse model. Environ Health Perspect 125:428-436; http://dx.doi.org/10.1289/EHP316.
Conflict of interest statement
The authors declare they have no actual or potential competing financial interests.
Figures
References
-
- Ambolet-Camoit A, Ottolenghi C, Leblanc A, Kim MJ, Letourneur F, Jacques S, et al. 2015. Two persistent organic pollutants which act through different xenosensors (alpha-endosulfan and 2,3,7,8 tetrachlorodibenzo-p-dioxin) interact in a mixture and downregulate multiple genes involved in human hepatocyte lipid and glucose metabolism. Biochimie 116 79 91, doi:10.1016/j.biochi.2015.07.003 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

