Secondary malignancies after partial versus whole breast irradiation: a systematic review and meta-analysis
- PMID: 27713125
- PMCID: PMC5342135
- DOI: 10.18632/oncotarget.12442
Secondary malignancies after partial versus whole breast irradiation: a systematic review and meta-analysis
Abstract
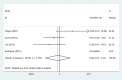
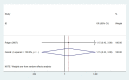
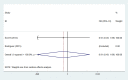
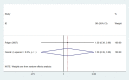
Secondary malignancies are a common complication for patients receiving radiotherapy. Here, we compared rates of secondary malignancies after partial breast irradiation (PBI) and whole breast irradiation (WBI). The MEDLINE, EMBASE, and the Cochrane Library databases were systematically searched to identify relevant randomized clinical trials comparing PBI with WBI in breast cancer patients treated with breast-conserving therapy. Four studies including 2,185 patients were selected. Compared to WBI, the pooled odds ratios (OR) for contralateral breast cancer were 0.86 (95% confidence interval (CI) 0.31-2.42; p = 0.78) after 5 years and 1.15 (95% CI 0.43-3.09; p = 0.78) after 10 years for PBI. The pooled ORs for secondary non-breast cancer were 0.91 (95% CI 0.49-1.67; p = 0.77) after 5 years and 1.20 (95% CI 0.39-3.66; p = 0.75) after 10 years for PBI compared to WBI. These results demonstrate that the risk of secondary malignancies is similar for PBI and WBI after breast-conserving radiotherapy.
Keywords: breast cancer; breast-conserving therapy; partial breast irradiation; secondary malignancies; whole breast irradiation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
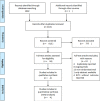
References
-
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-673667887-7. - DOI - PubMed
-
- Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–16. doi: 10.1016/S0140-673661629-2. - DOI - PMC - PubMed
-
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, Kamby C, Kjaer M, Gadeberg CC, Rasmussen BB, Blichert-Toft M, Mouridsen HT. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–8. doi: 10.1016/S0140-673609201-0. - DOI - PubMed
-
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen MB, Zedeler K. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–55. doi: 10.1056/NEJM199710023371401. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

