Sulfuretin promotes osteoblastic differentiation in primary cultured osteoblasts and in vivo bone healing
- PMID: 27713171
- PMCID: PMC5346641
- DOI: 10.18632/oncotarget.12460
Sulfuretin promotes osteoblastic differentiation in primary cultured osteoblasts and in vivo bone healing
Abstract
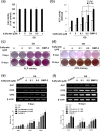
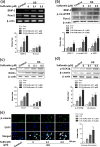
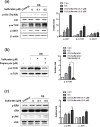
Although sulfuretin, the major flavonoid of Rhus verniciflua Stokes, has a variety of biological actions, its in vitro and in vivo effects on osteogenic potential remain poorly understood. The objective of the present study was to investigate the effects of sulfuretin on in vitro osteoblastic differentiation and the underlying signal pathway mechanisms in primary cultured osteoblasts and on in vivo bone formation using critical-sized calvarial defects in mice. Sulfuretin promoted osteogenic differentiation of primary osteoblasts, with increased ALP activity and mineralization, and upregulated differentiation markers, including ALP, osteocalcin, and osteopontin, in a concentration-dependent manner. The expression levels of Runx2, BMP-2, and phospho-Smad1/5/8 were upregulated by sulfuretin. Moreover, sulfuretin increased phosphorylation of Akt, mTOR, ERK, and JNK. Furthermore, sulfuretin treatment increased mRNA expression of Wnt ligands, phosphorylation of GSK3, and nuclear β-catenin protein expression. In vivo studies with calvarial bone defects revealed that sulfuretin significantly enhanced new bone formation by micro-computed tomography and histologic analysis. Collectively, these data suggest that sulfuretin acts through the activation of BMP, mTOR, Wnt/β-catenin, and Runx2 signaling to promote in vitro osteoblast differentiation and facilitate in vivo bone regeneration, and might be have therapeutic benefits in bone disease and regeneration.
Keywords: Differentiation; In vitro; In vivo; Osteoblasts; Osteogenesis; Signal pathways, Pathology Section.
Conflict of interest statement
The authors declare they have no competing financial interests.
Figures


References
-
- Andreopoulou P, Bockman RS. Management of postmenopausal osteoporosis. Annu Rev Med. 2015;66:329–342. - PubMed
-
- Nune KC, Kumar A, Misra RDK, Li SJ, Hao YL, Yang R. Osteoblast functions in functionally graded Ti-6Al-4V mesh structures. J Biomater Appl. 2016;30(8):1182–1204. - PubMed
-
- Nune KC, Kumar A, Murr LE, Misra RDK. Interplay between self-assembled structure of bone morphogenetic protein-2 (BMP-2) and osteoblast functions in three-dimensional titanium alloy scaffolds: Stimulation of osteogenic activity. J Biomed Mater Res A. 2016;104(2):517–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

