NSAIDs, Opioids, Cannabinoids and the Control of Pain by the Central Nervous System
- PMID: 27713305
- PMCID: PMC4033984
- DOI: 10.3390/ph3051335
NSAIDs, Opioids, Cannabinoids and the Control of Pain by the Central Nervous System
Abstract
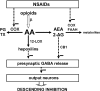
Nonsteroidal anti-inflammatory drugs (NSAIDs) act upon peripheral tissues and upon the central nervous system to produce analgesia. A major central target of NSAIDs is the descending pain control system. The rostral structures of the descending pain control system send impulses towards the spinal cord and regulate the transmission of pain messages. Key structures of the descending pain control system are the periaqueductal gray matter (PAG) and the rostral ventromedial region of the medulla (RVM), both of which are critical targets for endogenous opioids and opiate pharmaceuticals. NSAIDs also act upon PAG and RVM to produce analgesia and, if repeatedly administered, induce tolerance to themselves and cross-tolerance to opioids. Experimental evidence shows that this is due to an interaction of NSAIDs with endogenous opioids along the descending pain control system. Analgesia by NSAIDs along the descending pain control system also requires an activation of the CB1 endocannabinoid receptor. Several experimental approaches suggest that opioids, NSAIDs and cannabinoids in PAG and RVM cooperate to decrease GABAergic inhibition and thus enhance the descending flow of impulses that inhibit pain.
Keywords: NSAID; PAG; RVM; cannabinoid; descending pain control system; opioid.
Figures
Similar articles
-
Cannabinoids and Opioids Differentially Target Extrinsic and Intrinsic GABAergic Inputs onto the Periaqueductal Grey Descending Pathway.J Neurosci. 2022 Oct 12;42(41):7744-7756. doi: 10.1523/JNEUROSCI.0997-22.2022. Epub 2022 Sep 8. J Neurosci. 2022. PMID: 36414010 Free PMC article.
-
The Role of Cannabinoid Receptors in the Descending Modulation of Pain.Pharmaceuticals (Basel). 2010 Aug 16;3(8):2661-2673. doi: 10.3390/ph3082661. Pharmaceuticals (Basel). 2010. PMID: 27713370 Free PMC article. Review.
-
Metamizol, a non-opioid analgesic, acts via endocannabinoids in the PAG-RVM axis during inflammation in rats.Eur J Pain. 2012 May;16(5):676-89. doi: 10.1002/j.1532-2149.2011.00057.x. Epub 2011 Dec 19. Eur J Pain. 2012. PMID: 22337336
-
Endogenous opioid peptides in the descending pain modulatory circuit.Neuropharmacology. 2020 Aug 15;173:108131. doi: 10.1016/j.neuropharm.2020.108131. Epub 2020 May 15. Neuropharmacology. 2020. PMID: 32422213 Free PMC article. Review.
-
Descending modulation of pain: the GABA disinhibition hypothesis of analgesia.Curr Opin Neurobiol. 2014 Dec;29:159-64. doi: 10.1016/j.conb.2014.07.010. Epub 2014 Jul 26. Curr Opin Neurobiol. 2014. PMID: 25064178 Review.
Cited by
-
Stress-induced hyperalgesia instead of analgesia in patients with chronic musculoskeletal pain.Neurobiol Pain. 2022 Dec 6;13:100110. doi: 10.1016/j.ynpai.2022.100110. eCollection 2023 Jan-Jul. Neurobiol Pain. 2022. PMID: 36561877 Free PMC article.
-
Toward Composite Pain Biomarkers of Neuropathic Pain-Focus on Peripheral Neuropathic Pain.Front Pain Res (Lausanne). 2022 May 11;3:869215. doi: 10.3389/fpain.2022.869215. eCollection 2022. Front Pain Res (Lausanne). 2022. PMID: 35634449 Free PMC article. Review.
-
Periaqueductal Grey EP3 Receptors Facilitate Spinal Nociception in Arthritic Secondary Hypersensitivity.J Neurosci. 2016 Aug 31;36(35):9026-40. doi: 10.1523/JNEUROSCI.4393-15.2016. J Neurosci. 2016. PMID: 27581447 Free PMC article.
-
Tolerance effects of non-steroidal anti-inflammatory drugs microinjected into central amygdala, periaqueductal grey, and nucleus raphe: Possible cellular mechanism.Neural Regen Res. 2012 May 5;7(13):1029-39. doi: 10.3969/j.issn.1673-5374.2012.13.010. Neural Regen Res. 2012. PMID: 25722692 Free PMC article. Review.
-
Role of Keap1-Nrf2 Signaling in Anhedonia Symptoms in a Rat Model of Chronic Neuropathic Pain: Improvement With Sulforaphane.Front Pharmacol. 2018 Aug 8;9:887. doi: 10.3389/fphar.2018.00887. eCollection 2018. Front Pharmacol. 2018. PMID: 30135655 Free PMC article.
References
-
- Fields H.L. Pain. McGraw-Hill; New York, NY, USA: 1987.
-
- Fields H.L., Basbaum A.I. Brainstem control of spinal pain transmission neurons. Annu. Rev. Physiol. 1978;40:217–248. - PubMed
-
- Ren K., Dubner R. Enhanced descending modulation of nociception in rats with persistent hindpaw inflammation. J. Neurophysiol. 1996;76:3025–3037. - PubMed
-
- Schaible H.-G., Neugebauer V., Cervero F., Schmidt R.F. Changes in tonic descending inhibition of spinal neurons with articular input during the development of acute arthritis in the cat. J. Neurophysiol. 1991;66:1021–1032. - PubMed
-
- Danziger N., Weil-Fugazza J., Le Bars D., Bouhassira D. Stage-dependent changes in the modulation of spinal nociceptive neuronal activity during the course of inflammation. Eur. J. Neurosci. 2001;13:230–240. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

