Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets
- PMID: 27713405
- PMCID: PMC5059781
- DOI: 10.1038/ncomms13131
Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets
Abstract
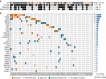
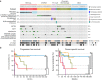
Renal cell carcinomas with unclassified histology (uRCC) constitute a significant portion of aggressive non-clear cell renal cell carcinomas that have no standard therapy. The oncogenic drivers in these tumours are unknown. Here we perform a molecular analysis of 62 high-grade primary uRCC, incorporating targeted cancer gene sequencing, RNA sequencing, single-nucleotide polymorphism array, fluorescence in situ hybridization, immunohistochemistry and cell-based assays. We identify recurrent somatic mutations in 29 genes, including NF2 (18%), SETD2 (18%), BAP1 (13%), KMT2C (10%) and MTOR (8%). Integrated analysis reveals a subset of 26% uRCC characterized by NF2 loss, dysregulated Hippo-YAP pathway and worse survival, whereas 21% uRCC with mutations of MTOR, TSC1, TSC2 or PTEN and hyperactive mTORC1 signalling are associated with better clinical outcome. FH deficiency (6%), chromatin/DNA damage regulator mutations (21%) and ALK translocation (2%) distinguish additional cases. Altogether, this study reveals distinct molecular subsets for 76% of our uRCC cohort, which could have diagnostic and therapeutic implications.
Figures


References
-
- Kovacs G. et al.. The Heidelberg classification of renal cell tumours. J. Pathol. 183, 131–133 (1997). - PubMed
-
- Eble J. N., Sauter G., Epstein J. I., Sesterhenn I. A. (eds). Tumours of the Urinary System and Male Genital Organs IARC Press (2004).
-
- Srigley J. R. et al.. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am. J. Surg. Pathol. 37, 1469–1489 (2013). - PubMed
-
- Zisman A. et al.. Unclassified renal cell carcinoma: clinical features and prognostic impact of a new histological subtype. J. Urol. 168, 950–955 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

