Zika virus infection during the period of maximal brain growth causes microcephaly and corticospinal neuron apoptosis in wild type mice
- PMID: 27713505
- PMCID: PMC5054421
- DOI: 10.1038/srep34793
Zika virus infection during the period of maximal brain growth causes microcephaly and corticospinal neuron apoptosis in wild type mice
Abstract
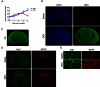
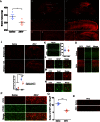
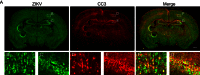
Zika virus (ZIKV) infection in pregnant women has been established as a cause of microcephaly in newborns. Here we test the hypothesis that neurodevelopmental stages when the brain is undergoing rapid growth are particularly vulnerable to the effects of ZIKV infection. We injected ZIKV intracranially into wild type C57BL/6 mice at two different time points: early postnatal development, when the brain is growing at its maximal rate, and at weaning, when the brain has largely reached adult size. Both time points showed widespread immunoreactivity for ZIKV and cleaved caspase 3 (CC3, a marker of apoptosis) throughout the brain. However, in early postnatal ZIKV injected mice, some brain areas and cell types display particularly large increases in apoptosis that we did not observe in older animals. Corticospinal pyramidal neurons, a cell type implicated in human microcephaly associated with ZIKV infection, are an example of one such cell type. Proliferating cells in the ventricular zone stem cell compartment are also depleted. These findings are consistent with the hypothesis that periods of rapid brain growth are especially susceptible to neurodevelopmental effects of ZIKV infection, and establish a valuable model to investigate mechanisms underlying neurodevelopmental effects of ZIKV infection and explore candidate therapeutics.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

