Safety and efficacy of sunitinib in patients from Latin America: subanalysis of an expanded access trial in metastatic renal cell carcinoma
- PMID: 27713637
- PMCID: PMC5045222
- DOI: 10.2147/OTT.S109445
Safety and efficacy of sunitinib in patients from Latin America: subanalysis of an expanded access trial in metastatic renal cell carcinoma
Abstract
Background: Sunitinib is an approved treatment for metastatic renal cell carcinoma (mRCC). The safety profile and efficacy of sunitinib were confirmed in a global expanded access trial (ClinicalTrials.gov identifier: NCT00130897). This report presents a subanalysis of the final trial data from patients in Latin America.
Methods: Treatment-naïve or previously treated mRCC patients aged ≥18 years received oral sunitinib at a starting dose of 50 mg/day on a 4-weeks-on/2-weeks-off schedule. Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent. Safety was assessed regularly, and tumor measurements were scheduled per local practice (using Response Evaluation Criteria in Solid Tumors).
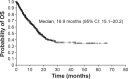
Results: In total, 348 patients from Latin America received sunitinib. Overall, 75% of patients had two or more sites of metastatic disease, 28% were aged ≥65 years, 14% had an Eastern Cooperative Oncology Group performance status ≥2, 9% had brain metastases, 9% had no prior nephrectomy, and 5% had non-clear cell RCC. Median treatment duration was 8 months, and median follow-up was 15.1 months. In total, 326 patients (94%) discontinued treatment, primarily due to death (41%) or lack of efficacy (22%). Most treatment-related adverse events were of mild to moderate severity (grade 1/2). Mucosal inflammation (reported in 54% of patients), diarrhea (53%), and asthenia (41%) were the most common any-grade treatment-related adverse events. Asthenia (12%), neutropenia (10%), and fatigue and thrombocytopenia (both 9%) were the most common grade 3/4 treatment-related adverse events. In total, 311 patients were included for tumor response, of whom eight (3%) had a complete response and 46 (15%) a partial response, yielding an objective response rate of 17%. Median duration of response, progression-free survival, and overall survival were 26.7, 12.1, and 16.9 months, respectively.
Conclusion: The efficacy and safety profile of sunitinib in patients with mRCC from Latin America was comparable to that in the entire cohort of the global expanded access trial.
Keywords: Latin America; expanded-access trial; kidney cancer; sunitinib; tyrosine kinase inhibitor.
Figures

References
-
- World Cancer Research Fund International Kidney cancer statistics [Internet] [Accessed July 25, 2014]. Available from: http://www.wcrf.org/cancer_statistics/data_specific_cancers/kidney_cance....
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. - PubMed
-
- Ljungberg B. Prognostic factors in renal cell carcinoma. Scand J Surg. 2004;93(2):118–125. - PubMed
-
- Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335(12):865–875. - PubMed
-
- Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24(1):25–35. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

