Postoperative prophylactic hepatic arterial infusion chemotherapy for stage III colorectal cancer: a retrospective study
- PMID: 27713643
- PMCID: PMC5045227
- DOI: 10.2147/OTT.S116815
Postoperative prophylactic hepatic arterial infusion chemotherapy for stage III colorectal cancer: a retrospective study
Abstract
Background: Radical resection is the main treatment for colorectal cancer (CRC), but metastasis or recurrence is common in which liver metastasis accounted for 83% of the cases. Therefore, the prognosis of patients with advanced CRC may be improved if liver metastasis is prevented. This study aims to investigate the efficacy of hepatic arterial infusion chemotherapy (HAIC) on liver metastases of stage III CRC patients after curative resection.
Methods: Between 2002 and 2008, 287 stage III CRC patients who had undergone radical resection were included in this study. According to postoperative adjuvant chemotherapy modality, these patients were divided into two groups. Patients in the combined therapy group received two cycles of HAIC plus four cycles of systemic chemotherapy, while patients in the monotherapy group received six cycles of systemic chemotherapy alone. The HAIC regimen consisted of hepatic arterial infusion of oxaliplatin (OXA, 85 mg/m2) on day 1 and 5-fluorouracil (5-FU, 2,400 mg/m2) on days 2 and 3 followed by a vein infusion of folinic acid (FA, 200 mg/m2) as a 2-hour infusion on days 2 and 3. The systemic chemotherapy regimen consisted of a 2-hour infusion of OXA (85 mg/m2) on day 1 followed by FA (200 mg/m2) as a 2-hour infusion on days 2 and 3, and by 5-FU (2,400 mg/m2) as a 48-hour infusion. This was repeated every 4 weeks. All cases were followed up for 5 years or until death. The 5-year overall survival, disease-free survival, liver metastases-free survival, and the overall liver metastases rates were retrospectively compared.
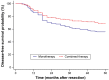
Results: Significant differences were found in the 5-year overall survival (combined therapy, 70.71%; monotherapy, 57.14%; P=0.014), disease-free survival (combined therapy, 69.29%; monotherapy, 55.78%; P=0.021), and liver metastases-free survival rates (combined therapy, 70%; monotherapy, 56.46%; P=0.019).
Conclusion: Prophylactic adjuvant HAIC can prevent metachronous liver metastases and improve the prognosis of patients with stage III CRC after curative resection.
Keywords: chemotherapy; colorectal cancer; hepatic arterial infusion chemotherapy; liver metastases.
Figures


References
-
- Yao Y, Zhao H, Sun Y, Lin F, Tang L, Chen P. Combined chemotherapy of hydroxycampothecin with oxaliplatin as an adjuvant treatment for human colorectal cancer. Tohoku J Exp Med. 2008;215(3):267–278. - PubMed
-
- Weiss L, Grundmann E, Torhorst J, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Patho. 1986;150(3):195–203. - PubMed
-
- Piedbois P, Buyse M, Kemeny N, et al. Meta-Analysis Group in Cancer Reappraisal of hepatic arterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. J Natl Cancer Inst. 1996;88(5):252–258. - PubMed
-
- Ota M, Shimada H, Masui H, et al. Adjuvant hepatic arterial infusion chemotherapy after curative resection for Dukes C colorectal cancer: a pilot study. Hepatogastroenterology. 2004;51(55):124–127. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

