An Introduction to Survival Statistics: Kaplan-Meier Analysis
- PMID: 27713848
- PMCID: PMC5045282
- DOI: 10.6004/jadpro.2016.7.1.8
An Introduction to Survival Statistics: Kaplan-Meier Analysis
Figures
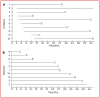
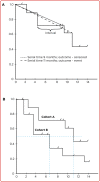

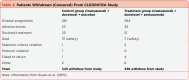
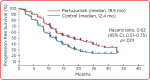
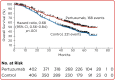

References
-
- Baselga José, Cortés Javier, Kim Sung-Bae, Im Seock-Ah, Hegg Roberto, Im Young-Hyuck, Roman Laslo, Pedrini José Luiz, Pienkowski Tadeusz, Knott Adam, Clark Emma, Benyunes Mark C, Ross Graham, Swain Sandra M. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. The New England journal of medicine. 2012;366:109–119. - PMC - PubMed
-
- Bollschweiler Elfriede. Benefits and limitations of Kaplan-Meier calculations of survival chance in cancer surgery. Langenbeck’s archives of surgery / Deutsche Gesellschaft für Chirurgie. 2003;388:239–244. - PubMed
-
- Brant J M, Beck S. L, Dudley W N, Cobb P, Pepper G, Miaskowski C. Symptom trajectories in posttreatment cancer survivors. . Cancer Nursing. 2011;34(1):67–77. - PubMed
-
- Dudley William N, McGuire Deborah B, Peterson Douglas E, Wong Bob. Application of multilevel growth-curve analysis in cancer treatment toxicities: the exemplar of oral mucositis and pain. Oncology nursing forum. 2009;36:E11–19. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
