ERK5 and Cell Proliferation: Nuclear Localization Is What Matters
- PMID: 27713878
- PMCID: PMC5031611
- DOI: 10.3389/fcell.2016.00105
ERK5 and Cell Proliferation: Nuclear Localization Is What Matters
Abstract
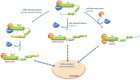
ERK5, the last MAP kinase family member discovered, is activated by the upstream kinase MEK5 in response to growth factors and stress stimulation. MEK5-ERK5 pathway has been associated to different cellular processes, playing a crucial role in cell proliferation in normal and cancer cells by mechanisms that are both dependent and independent of its kinase activity. Thus, nuclear ERK5 activates transcription factors by either direct phosphorylation or acting as co-activator thanks to a unique transcriptional activation TAD domain located at its C-terminal tail. Consequently, ERK5 has been proposed as an interesting target to tackle different cancers, and either inhibitors of ERK5 activity or silencing the protein have shown antiproliferative activity in cancer cells and to block tumor growth in animal models. Here, we review the different mechanisms involved in ERK5 nuclear translocation and their consequences. Inactive ERK5 resides in the cytosol, forming a complex with Hsp90-Cdc37 superchaperone. In a canonical mechanism, MEK5-dependent activation results in ERK5 C-terminal autophosphorylation, Hsp90 dissociation, and nuclear translocation. This mechanism integrates signals such as growth factors and stresses that activate the MEK5-ERK5 pathway. Importantly, two other mechanisms, MEK5-independent, have been recently described. These mechanisms allow nuclear shuttling of kinase-inactive forms of ERK5. Although lacking kinase activity, these forms activate transcription by interacting with transcription factors through the TAD domain. Both mechanisms also require Hsp90 dissociation previous to nuclear translocation. One mechanism involves phosphorylation of the C-terminal tail of ERK5 by kinases that are activated during mitosis, such as Cyclin-dependent kinase-1. The second mechanism involves overexpression of chaperone Cdc37, an oncogene that is overexpressed in cancers such as prostate adenocarcinoma, where it collaborates with ERK5 to promote cell proliferation. Although some ERK5 kinase inhibitors have shown antiproliferative activity it is likely that those tumors expressing kinase-inactive nuclear ERK5 will not respond to these inhibitors.
Keywords: Cdc37; ERK5; Hsp90; MAP kinase; cancer; cell proliferation; nuclear translocation; transcriptional co-activator.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

