Role of CFTR in epithelial physiology
- PMID: 27714410
- PMCID: PMC5209439
- DOI: 10.1007/s00018-016-2391-y
Role of CFTR in epithelial physiology
Abstract
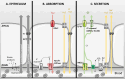
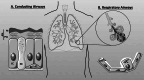
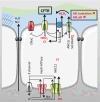
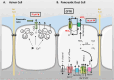
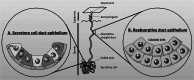
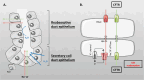
Salt and fluid absorption and secretion are two processes that are fundamental to epithelial function and whole body fluid homeostasis, and as such are tightly regulated in epithelial tissues. The CFTR anion channel plays a major role in regulating both secretion and absorption in a diverse range of epithelial tissues, including the airways, the GI and reproductive tracts, sweat and salivary glands. It is not surprising then that defects in CFTR function are linked to disease, including life-threatening secretory diarrhoeas, such as cholera, as well as the inherited disease, cystic fibrosis (CF), one of the most common life-limiting genetic diseases in Caucasian populations. More recently, CFTR dysfunction has also been implicated in the pathogenesis of acute pancreatitis, chronic obstructive pulmonary disease (COPD), and the hyper-responsiveness in asthma, underscoring its fundamental role in whole body health and disease. CFTR regulates many mechanisms in epithelial physiology, such as maintaining epithelial surface hydration and regulating luminal pH. Indeed, recent studies have identified luminal pH as an important arbiter of epithelial barrier function and innate defence, particularly in the airways and GI tract. In this chapter, we will illustrate the different operational roles of CFTR in epithelial function by describing its characteristics in three different tissues: the airways, the pancreas, and the sweat gland.
Keywords: Bicarbonate; CFTR; Chloride; Epithelial transport; Physiology.
Conflict of interest statement
Compliance with ethical standards Funding Work from the authors is supported by a Strategic Research Centre Grant (INOVCF) from the CF Trust UK (SRC003).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

