Role of oxidative stress in oxaliplatin-induced enteric neuropathy and colonic dysmotility in mice
- PMID: 27714760
- PMCID: PMC5120153
- DOI: 10.1111/bph.13646
Role of oxidative stress in oxaliplatin-induced enteric neuropathy and colonic dysmotility in mice
Abstract
Background and purpose: Oxaliplatin is a platinum-based chemotherapeutic drug used as a first-line therapy for colorectal cancer. However, its use is associated with severe gastrointestinal side-effects resulting in dose limitations and/or cessation of treatment. In this study, we tested whether oxidative stress, caused by chronic oxaliplatin treatment, induces enteric neuronal damage and colonic dysmotility.
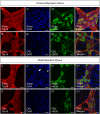
Experimental approach: Oxaliplatin (3 mg·kg-1 per day) was administered in vivo to Balb/c mice intraperitoneally three times a week. The distal colon was collected at day 14 of treatment. Immunohistochemistry was performed in wholemount preparations of submucosal and myenteric ganglia. Neuromuscular transmission was studied by intracellular electrophysiology. Circular muscle tone was studied by force transducers. Colon propulsive activity studied in organ bath experiments and faeces were collected to measure water content.
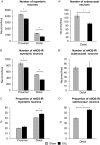
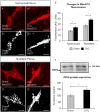
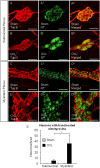
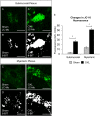
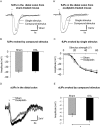
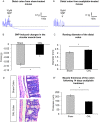
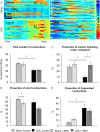
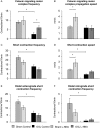
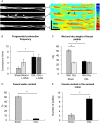
Key results: Chronic in vivo oxaliplatin treatment resulted in increased formation of reactive oxygen species (O2 -), nitration of proteins, mitochondrial membrane depolarisation resulting in the release of cytochrome c, loss of neurons, increased inducible NOS expression and apoptosis in both the submucosal and myenteric plexuses of the colon. Oxaliplatin treatment enhanced NO-mediated inhibitory junction potentials and altered the response of circular muscles to the NO donor, sodium nitroprusside. It also reduced the frequency of colonic migrating motor complexes and decreased circular muscle tone, effects reversed by the NO synthase inhibitor, Nω-Nitro-L-arginine.
Conclusion and implications: Our study is the first to provide evidence that oxidative stress is a key player in enteric neuropathy and colonic dysmotility leading to symptoms of chronic constipation observed in oxaliplatin-treated mice.
© 2016 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures
References
-
- André T, Boni C, Mounedji‐Boudiaf L, Navarro M, Tabernero J, Hickish T et al. (2004). Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350: 2343–2351. - PubMed
-
- Beckman JS, Koppenol WH (1996). Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271: C1424–C1437. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

