Trastuzumab cardiotoxicity: from clinical trials to experimental studies
- PMID: 27714776
- PMCID: PMC5647179
- DOI: 10.1111/bph.13643
Trastuzumab cardiotoxicity: from clinical trials to experimental studies
Abstract
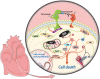
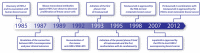
Epidermal growth factor receptor-2 (HER-2) is overexpressed in 20 to 25% of human breast cancers, which is associated with aggressive tumour growth and poor prognosis. Trastuzumab (Herceptin®) is a humanized monoclonal antibody directed against HER-2, the first highly selective form of therapy targeting HER-2 overexpressing tumours. Although initial trials indicated high efficacy and a favourable safety profile of the drug, the first large, randomized trial prompted a retrospective analysis of cardiac dysfunction in earlier trials utilizing trastuzumab. There has been ongoing debate on the cardiac safety of trastuzumab ever since, initiating numerous clinical and preclinical investigations to better understand the background of trastuzumab cardiotoxicity and evaluate its effects on patient morbidity. Here, we have given a comprehensive overview of our current knowledge on the cardiotoxicity of trastuzumab, primarily focusing on data from clinical trials and highlighting the main molecular mechanisms proposed.
Linked articles: This article is part of a themed section on New Insights into Cardiotoxicity Caused by Chemotherapeutic Agents. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.21/issuetoc.
Published 2016. This article is a U.S. Government work and is in the public domain in the USA.
Figures
References
-
- Amadori D, Milandri C, Comella G, Saracchini S, Salvagni S, Barone C et al. (2011). A phase I/II trial of non‐pegylated liposomal doxorubicin, docetaxel and trastuzumab as first‐line treatment in HER‐2‐positive locally advanced or metastatic breast cancer. Eur J Cancer 47: 2091–2098. - PubMed
-
- Anton A, Ruiz A, Plazaola A, Calvo L, Segui MA, Santaballa A et al. (2011). Phase II clinical trial of liposomal‐encapsulated doxorubicin citrate and docetaxel, associated with trastuzumab, as neoadjuvant treatment in stages II and IIIA HER2‐overexpressing breast cancer patients. GEICAM 2003‐03 study. Ann Oncol 22: 74–79. - PubMed
-
- Aogi K, Saeki T, Nakamura S, Kashiwaba M, Sato N, Masuda N et al. (2013). A multicenter, phase II study of epirubicin/cyclophosphamide followed by docetaxel and concurrent trastuzumab as primary systemic therapy for HER‐2 positive advanced breast cancer (the HER2NAT study). Int J Clin Oncol 18: 598–606. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

