KSHV reduces autophagy in THP-1 cells and in differentiating monocytes by decreasing CAST/calpastatin and ATG5 expression
- PMID: 27715410
- PMCID: PMC5173267
- DOI: 10.1080/15548627.2016.1235122
KSHV reduces autophagy in THP-1 cells and in differentiating monocytes by decreasing CAST/calpastatin and ATG5 expression
Abstract
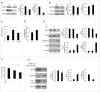
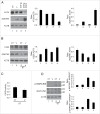
We have previously shown that Kaposi sarcoma-associated herpesvirus (KSHV) impairs monocyte differentiation into dendritic cells (DCs). Macroautophagy/autophagy has been reported to be essential in such a differentiating process. Here we extended these studies and found that the impairment of DC formation by KSHV occurs through autophagy inhibition. KSHV indeed reduces CAST (calpastatin) and consequently decreases ATG5 expression in both THP-1 monocytoid cells and primary monocytes. We unveiled a new mechanism put in place by KSHV to escape from immune control. The discovery of viral immune suppressive strategies that contribute to the onset and progression of viral-associated malignancies is of fundamental importance for finding new therapeutic approaches against them.
Keywords: ATG5; CAPN/calpain; CAST/calpastatin; KSHV; MAPK/JNK; SQSTM1/p62; THP-1; autophagy; dendritic cells; herpesvirus; monocytes.
Figures




References
-
- Jin M, Klionsky DJ. Regulation of autophagy: modulation of the size and number of autophagosomes. FEBS Lett 2014; 588:2457-63; PMID:24928445; http://dx.doi.org/ 10.1016/j.febslet.2014.06.015 - DOI - PMC - PubMed
-
- Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 2014; 20:460-73; PMID:23725295; http://dx.doi.org/ 10.1089/ars.2013.5371 - DOI - PMC - PubMed
-
- Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell 2014; 159:1263-76; PMID:25480292; http://dx.doi.org/ 10.1016/j.cell.2014.11.006 - DOI - PMC - PubMed
-
- Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res 2014; 24:24-41; PMID:24366339; http://dx.doi.org/ 10.1038/cr.2013.168 - DOI - PMC - PubMed
-
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008; 132:27-42; PMID:18191218; http://dx.doi.org/ 10.1016/j.cell.2007.12.018 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
