Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA) - protocol of a randomized phase II trial (NOA 10/ARO 2013-1)
- PMID: 27716184
- PMCID: PMC5052714
- DOI: 10.1186/s12885-016-2806-z
Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA) - protocol of a randomized phase II trial (NOA 10/ARO 2013-1)
Abstract
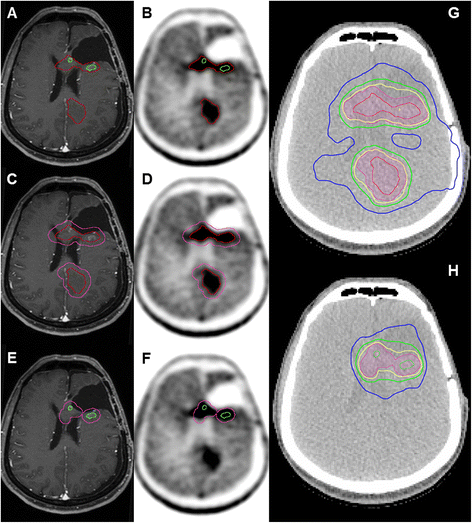
Background: The higher specificity of amino-acid positron emission tomography (AA-PET) in the diagnosis of gliomas, as well as in the differentiation between recurrence and treatment-related alterations, in comparison to contrast enhancement in T1-weighted MRI was demonstrated in many studies and is the rationale for their implementation into radiation oncology treatment planning. Several clinical trials have demonstrated the significant differences between AA-PET and standard MRI concerning the definition of the gross tumor volume (GTV). A small single-center non-randomized prospective study in patients with recurrent high grade gliomas treated with stereotactic fractionated radiotherapy (SFRT) showed a significant improvement in survival when AA-PET was integrated in target volume delineation, in comparison to patients treated based on CT/MRI alone.
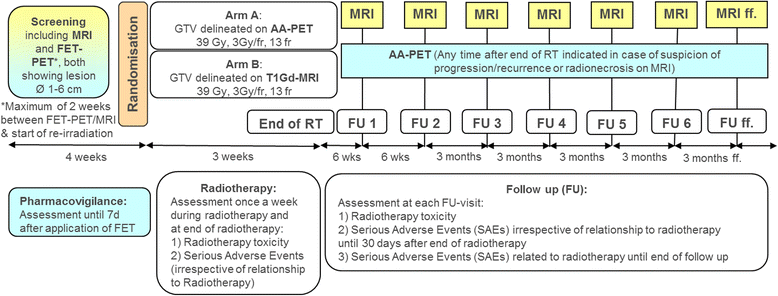
Methods: This protocol describes a prospective, open label, randomized, multi-center phase II trial designed to test if radiotherapy target volume delineation based on FET-PET leads to improvement in progression free survival (PFS) in patients with recurrent glioblastoma (GBM) treated with re-irradiation, compared to target volume delineation based on T1Gd-MRI. The target sample size is 200 randomized patients with a 1:1 allocation ratio to both arms. The primary endpoint (PFS) is determined by serial MRI scans, supplemented by AA-PET-scans and/or biopsy/surgery if suspicious of progression. Secondary endpoints include overall survival (OS), locally controlled survival (time to local progression or death), volumetric assessment of GTV delineated by either method, topography of progression in relation to MRI- or PET-derived target volumes, rate of long term survivors (>1 year), localization of necrosis after re-irradiation, quality of life (QoL) assessed by the EORTC QLQ-C15 PAL questionnaire, evaluation of safety of FET-application in AA-PET imaging and toxicity of re-irradiation.
Discussion: This is a protocol of a randomized phase II trial designed to test a new strategy of radiotherapy target volume delineation for improving the outcome of patients with recurrent GBM. Moreover, the trial will help to develop a standardized methodology for the integration of AA-PET and other imaging biomarkers in radiation treatment planning.
Trial registration: The GLIAA trial is registered with ClinicalTrials.gov ( NCT01252459 , registration date 02.12.2010), German Clinical Trials Registry ( DRKS00000634 , registration date 10.10.2014), and European Clinical Trials Database (EudraCT-No. 2012-001121-27, registration date 27.02.2012).
Keywords: Amino-acid PET; Re-irradiation; Recurrent glioblastoma; T1-Gd-MRI.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

