Sarilumab plus methotrexate suppresses circulating biomarkers of bone resorption and synovial damage in patients with rheumatoid arthritis and inadequate response to methotrexate: a biomarker study of MOBILITY
- PMID: 27716324
- PMCID: PMC5052933
- DOI: 10.1186/s13075-016-1132-9
Sarilumab plus methotrexate suppresses circulating biomarkers of bone resorption and synovial damage in patients with rheumatoid arthritis and inadequate response to methotrexate: a biomarker study of MOBILITY
Abstract
Background: Interleukin 6 (IL-6) signaling plays a key role in the pathophysiology of rheumatoid arthritis (RA) and is inhibited by sarilumab, a human monoclonal antibody blocking the IL-6 receptor alpha (IL-6Rα). The effects of sarilumab plus methotrexate (MTX) on serum biomarkers of joint damage and bone resorption were assessed in two independent studies (phase II (part A) and phase III (part B)) of patients with RA with a history of inadequate response to MTX from the MOBILITY study (NCT01061736).
Methods: Serum samples were analyzed at baseline and prespecified posttreatment time points. Biomarkers of tissue destruction, cartilage degradation, and synovial inflammation were measured in part A; assessment of these markers was repeated in part B and included additional analysis of biomarkers of bone formation and resorption (including soluble receptor activator of nuclear factor-kB ligand (sRANKL)). A mixed model for repeated measures was used to compare treatment effects on change in biomarkers. Additionally, changes from baseline in biomarkers were compared between American College of Rheumatology 50 % responders and nonresponders and between patients who achieved or did not achieve low disease activity (LDA), separately by treatment group, at week 24.
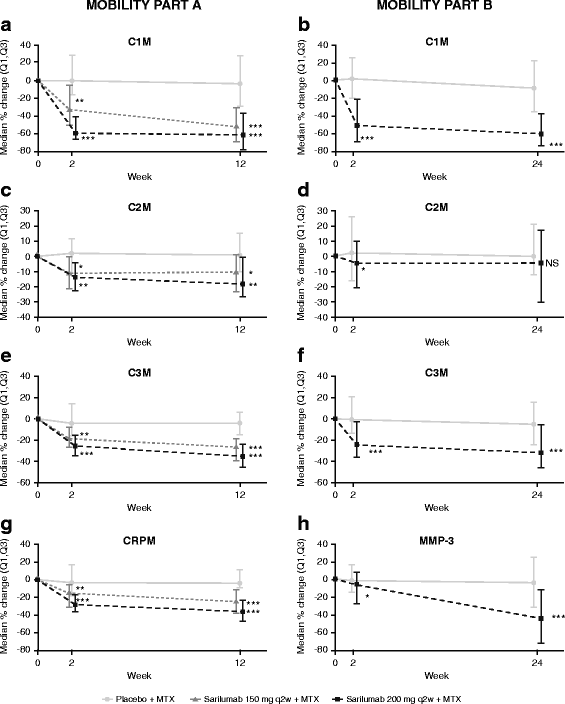
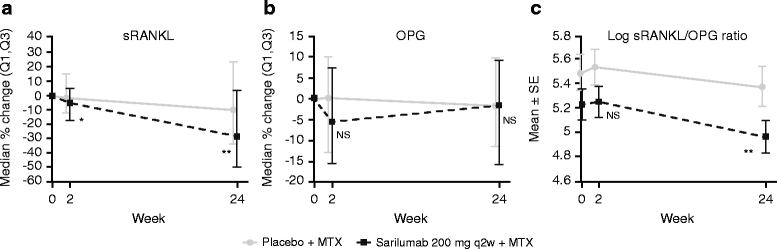
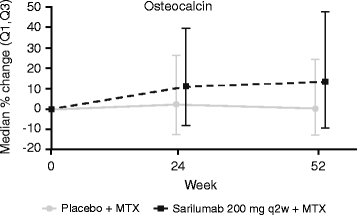
Results: In part A, sarilumab 150 and 200 mg every 2 weeks (q2w) significantly reduced biomarkers of tissue destruction, cartilage degradation, and synovial inflammation at both 2 and 12 weeks posttreatment (p < 0.05 vs placebo). These results were replicated in part B, with markers of these damaging processes reduced at weeks 2 and 24 (p < 0.05 vs placebo). Additionally, sarilumab 200 mg q2w significantly reduced both sRANKL and sRANKL/osteoprotegerin ratio at week 24 (p < 0.01 vs placebo). Trends for reduction were noted for several biomarkers in patients who achieved LDA compared with those who did not.
Conclusions: Sarilumab plus MTX significantly suppressed biomarkers of bone resorption and joint damage, as compared with placebo plus MTX, in patients with RA. Additional work is needed to determine whether differences in biomarker profiles at baseline or posttreatment can identify patients who achieve improvement in disease activity.
Trial registration: ClinicalTrials.gov, NCT01061736 , February 2, 2010.
Keywords: Biomarker; RANKL/OPG; Rheumatoid arthritis; Sarilumab.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

