Toll-like receptor 2 (TLR2) plays a role in controlling cutaneous leishmaniasis in vivo, but does not require activation by parasite lipophosphoglycan
- PMID: 27716391
- PMCID: PMC5053327
- DOI: 10.1186/s13071-016-1807-8
Toll-like receptor 2 (TLR2) plays a role in controlling cutaneous leishmaniasis in vivo, but does not require activation by parasite lipophosphoglycan
Abstract
Background: Leishmaniasis is a neglected tropical disease affecting millions of individuals worldwide. Despite several studies reporting involvement of the innate immune receptor Toll-like receptor 2 (TLR2) in the recognition of surface glycolipids from Leishmania parasites in vitro, the role of TLR2 and its co-receptors during cutaneous leishmaniasis infection in vivo is unknown.
Methods: To explore the role of TLR2 and its co-receptors in cutaneous leishmaniasis, mice deficient in either TLR2, 4, 1 or 6, or wild-type (WT) controls, were infected with either Leishmania major promastigotes, L. mexicana promastigotes, L. mexicana amastigotes, or LPG1 -/- L. mexicana promastigotes. For each infection, lesion sizes were monitored and parasite burden was assessed at various time points. To assess immune responses, draining lymph node (DLN) cells were re-stimulated with parasite antigens and the production of cytokines and parasite-specific antibody isotypes in blood was determined by ELISA.
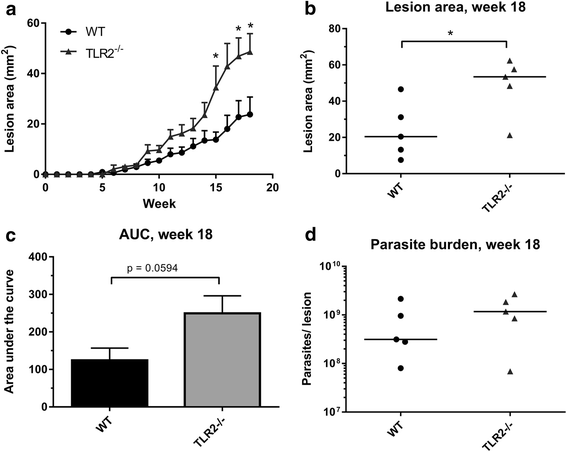
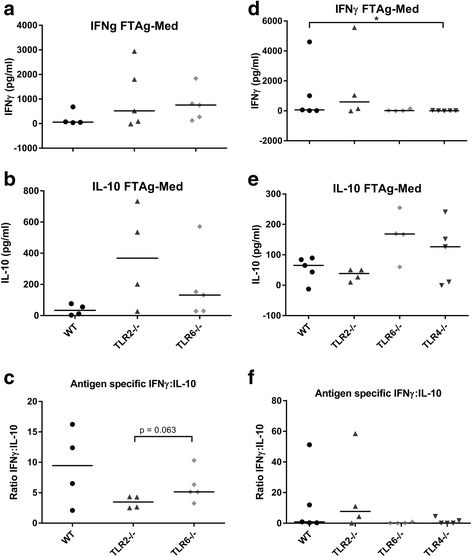
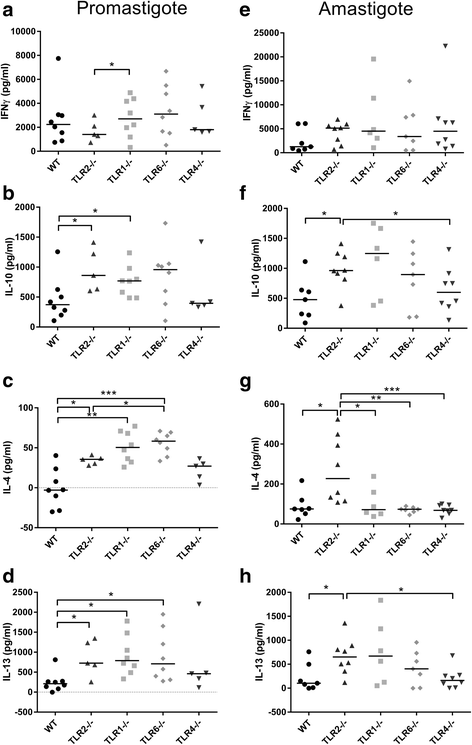
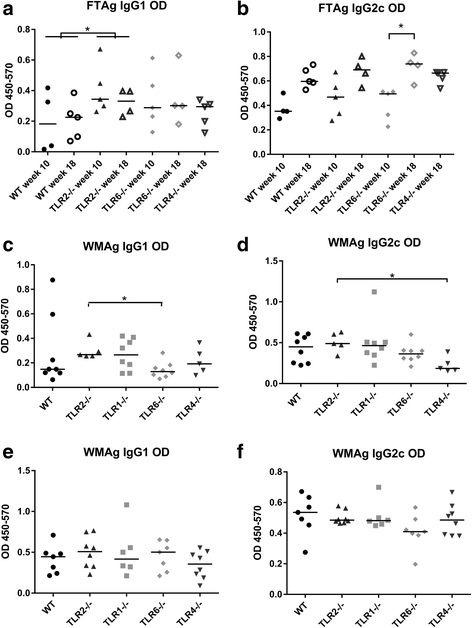
Results: Mice deficient in TLR2 and TLR4 presented with larger lesions and higher parasite burdens than WT controls. Mice lacking TLR2 co-receptors TLR1 or TLR6 did not show exacerbated infection, suggesting that TLR2 does not require either co-receptor in the recognition of Leishmania infection. Furthermore, it appears that lipophosphoglycan (LPG) is not the major mediator of TLR2 activation during infection with L. mexicana, as parasites lacking LPG (axenic amastigotes and LPG1 -/- promastigotes) also resulted in exacerbated disease in TLR2-/- mice. Infected TLR2-/- mice show a skewed Th2 immune response to Leishmania parasites, as demonstrated by elevated IL-4, IL-13 and IL-10 production by DLN cells from L. mexicana infected mice in response to antigen. Furthermore, L. major infected TLR2-/- mice have elevated antigen-specific IgG1 antibodies.
Conclusions: TLR2 deficiency leads to exacerbation of disease and parasite burden through promotion of Th2 immunity. TLR2 activation in vivo occurs independently of parasite LPG, suggesting other parasite ligands are involved in TLR2 recognition of Leishmania.
Keywords: Leishmania major; Leishmania mexicana; Lipophosphoglycan; TLR2.
Figures


References
-
- WHO . WHO Technical Report Series. Geneva: WHO; 2010. Control of the leishmaniases: Report of a meeting of the WHO Expert Committee on the control of leishmaniases.
-
- Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147(9):3149–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

